Abstract
During the past decades, advancement in pubertal maturation in children has been noticed worldwide. Growing evidence indicates that increasing prevalence of obesity in children is a major factor for the secular trend of earlier puberty. In girls, several epidemiologic studies suggest that earlier pubertal onset and earlier menarche might be caused by obesity. On the other hand, in boys, few research reported an association between obesity and pubertal development, and the results are inconsistent; Some studies found a link between obesity and delayed puberty, but others reported a causal relationship between obesity and early puberty. To date, mechanisms linking childhood obesity and earlier puberty remain unclear. In this review, we presented the potential impact of obesity on puberty-related hormones and summarized human studies on potential relationship of childhood adiposity and pubertal development.
Puberty is a complex process during which children attain secondary sexual characteristics and reproductive capability. Normal puberty is initiated by reactivation of the hypothalamic secretion of gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to release follicle stimulating hormone (FSH) and luteinizing hormone (LH), in turn, FSH and LH stimulates the gonadal development and sex steroid production. The first sign of pubertal onset is an increase in testicular volume above 4 mL (Tanner G2 stage) in boys and the first appearance of breast buds (Tanner B2 stage) in girls [1]. A number of factors including ethnicity, inheritance, stress, body fat and nutrition are known to be involved in the timing and tempo of pubertal development.
During the past decades, advancement in the age of pubertal development in girls has been reported all around the world. The age at thelarche has advanced about one year since mid-1980s and early 1990s among US white girls [2,3] and Danish girls [4]. With regards to menarche, trend toward earlier menarche is continuing in China [5] and Korea [6] since 1980s, meanwhile decrease in menarcheal age appears to be slowed down in developed nations such as US [7] Japan [8] and some European countries [9,10]. Also in boys, secular trend of earlier pubertal onset has been noted in US [11], Danmark [12], Korea [13], and China [14]. Interestingly, these secular trends of puberty have coincided with the worldwide increase in childhood obesity prevalence. A rapid increase of obesity prevalence in children has been noted in many countries including US [15], UK [16], Germany [17], France [17], Austrailia [18], Brazil [19], China [19], and Japan [20]. In Korea, the prevalence of childhood obesity increased from 5.8% in 1997 to 9.7% in 2005 [21], and now it has reached 10-20% and among high schoolers [22]. Several epidemiologic studies have demonstrated that girls with greater body mass index (BMI) enter thelarche [5,23,24] or menarche [25] earlier than girls with normal BMI, leading to a suggestion that excess adiposity influence the onset and progression of puberty.
In this paper, we reviewed the possible mechanism by which obesity may influence pubertal maturation in children and summarized human studies showing impact of childhood obesity on the pubertal timing.
In 1970, Frisch and Revelle [26] proposed a hypothesis that a weight of 48 kg is required to achieve menarche, suggesting that the critical weight causes a change in metabolic rate, in turn, affects hypothalamus-ovarian feedback. This hypothesis has been supported by the discovery of leptin, a hormone secreted by adipocytes. Leptin informs hypothalamus of amount of body fat mass, and suppresses appetite and stimulates energy expenditure [27]. Also, leptin play a role in pubertal development [27]. Leptin deficient mice have obese phenotype and fail to achieve puberty and fertility due to hypogonadotropic hypogonadism [28], and their infertility is reversed by the administration of leptin [29]. Similarly, human individuals with mutations in the leptin or leptin receptor gene have hypogonadotropic hypogonadism [30,31], and these individuals gain LH pulsatility of puberty following leptin administration [32,33]. However, several evidences indicate that leptin is a permissive factor for pubertal onset but is not a trigger factor for timing of puberty. For instance, leptin administration to normal prepubertal mice does not induce precocious puberty [34], and serum leptin levels in rodents remain relatively constant during the prepubertal and postpubertal stages [35]. Longitudinal human studies showed that serum leptin levels increase gradually during peripubertal period, rather than surging at the pubertal onset [36,37]. Mechanism of leptin action on pubertal onset is explained as an activation of GnRH-gonadotropin axis. Leptin receptors have been identified in the anterior pituitary [38] and leptin directly stimulates the release of LH and FSH [39]. Leptin receptors are also expressed in ventral premammillary neurons (PMV) which expresses the excitory neurotransmitter glutamate [40]. Leptin stimulates PMV to release glutamate, which, in turn, activates GnRH neurons (Fig. 1) [41].
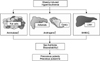
During puberty, increased growth hormone induces insulin resistance and physiological hyperinsulinemia [42]. Increased insulin facilitates pubertal weight gain, vise versa, obesity exacerbates the pubertal insulin resistance [43]. Obesity induced hyperinsulinemia may exaggerate pubertal maturation via various endocrine pathways. First, hyperinsulinemia in obesity may stimulate adrenal androgen secretion and precocious pubarche [44,45]. Low birth weight and rapid postnatal weight gain has been demonstrated to increase subsequent obesity risk [46] and linked to precocious pubarche and subsequent early puberty [47,48]. Studies reporting insulin sensitizing treatment (with metformin) prevents early menarche in girls with precocious pubarche [49,50] suggests important role of hyperinsulinemia in pubertal maturation. Second, hyperinsulinemia can reduce sex hormone binding globulin (SHBG) production from liver [51], increasing sex steroid bioavailability. Third, insulin stimulates ovarian growth and steroidogenesis via insulin receptors in ovary, increasing production of estrogens and androgens [52]. Fourth, aromatase activity is increased in obese children, possibly by increased insulin, resulting in increased conversion of androgens to estrogens [43]. Increased sex steroid levels by insulin effects on multiple organs could induce gonadotropin-independent or gonadotropin-dependent precocious puberty [53] (Fig. 2).
A number of evidences have demonstrated an associations between adiposity and early puberty in girls (Table 1). In 1997, Pediatric Research in Office Settings (PROS) study demonstrated that girls aged 6-9 years with breast development had higher BMI z-scores compared with prepubertal girls at each age [23]. Similarly, a study on pubertal development in black girls aged 8-10 years reported that increasing stages of breast development were related to increased BMI, waist circumference and fat mass [54]. A nationally representative study based on the data from the National Health Examination Survey (NHES 1963-1970) and National Health And Nutrition Examination Survey III (NHANES 1988-1944) was examined the trend of menarcheal age during the 25 year period [55]. The authors reported that menarcheal age advanced from 12.7 to 12.5 years and prevalence of overweight increased from 16% to 27% during the study period. And they also demonstrated that the average menarcheal age in 1988-1994 could be predictable based on the secular changes in distributions of BMI z-score, race, and age, suggesting causality exists between increasing obesity and advanced menarcheal age [55]. Other studies using NHANES III data demonstrated that prevalence of obesity and overweight is significantly higher in girls with early puberty than in control [24], vise versa, early puberty and early menarche are more prevalent in overweight girls than in normal weight girls [56]. Besides cross-sectional studies, there are some longitudinal studies that investigated the association of body composition and pubertal timing in girls. The Bolgalusa Heart study, which examined the relations between menarche and prepubertal adiposity, found that increased BMI z-score measured at aged of 5-9.9 years correlates earlier age (<11.0 years) at menarche [25]. A cohort study in Australia reported that overweight at age 5 years is a predictor for more advanced pubertal stages at age 14 years [57]. In a prospective study in Germany, higher prepubertal BMI was associated with earlier pubertal progression and shorter puberty duration, resulting earlier attainment of menarche [58]. Likewise, a recent prospective study demonstrated that fat mass at age 8 years was associated with advanced pubertal stage at age 11 years and BMI at age 11 years had negative association with the age of menarche [59]. As discussed earlier, small size at birth and childhood catch-up growth have been associated with increased risk of premature pubarche and early menarche, resulting from insulin resistance [48,49]. Although a large number of studies evaluated the association between adiposity and pubertal stage on physical examination, the relationship between adiposity and precocious puberty diagnosed with hormonal assay has been rarely discussed. Recently, we demonstrated that girls with precocious puberty has higher adiposity than control, and adiposity and central obesity is severer in girls with gonadotropin-independent precocious puberty puberty than in girls with gonadotropin-dependent precocious puberty [60]. This supports that obesity is a contributing factor for early puberty especially in girls with gonadotropin-independent precocious puberty.
There are fewer studies on a relationship between obesity and pubertal maturation in boys (Table 2). Contrary to the study results reporting significant associations between obesity and earlier puberty in girls, the relationship is mixed in boys according to the population studied. In a cross-sectional study in Italy, the obese boys tended to have delayed pubertal maturation [61]. Similarly, a large scaled US study based on NHANES III data, pubertal development was delayed in obese boys [24]. A more recent prospective study in US showed that boys in the highest BMI trajectory had a higher risk of later pubertal onset [62]. Putative mechanism of delayed puberty in obese boys was explained as increased circulating estrogens by increased aromatase activity in fat tissue. Increased estrogens, in turn, may inhibit gonadotropin secretion in negative feedback mechanism [63]. However, some longitudinal studies have demonstrated a positive relationships between pubertal onset and obesity in boys. A Spanish study [64] and an Australian study [57] reported a nagative relation between the age of pubertal onset and prepubertal BMI in boys. A Swedish population-based study [65] also demonstrated similar impact of childhood BMI on early pubertal onset and reduced height gain during puberty. A UK cohort study [48] showed low birth weight and catch-up growth increased the risk of premature pubarche in boys, as well in girls. Finally, the Copenhagen Puberty Study [12] evaluated the causal effect of increased adiposity on secular trends in earlier pubertal onset among Danish boys. The age at pubertal onset has decreased, whereas BMI z-score has increased between 1991-1993 and 2006-2008. Interestingly, after adjustment for BMI, the difference in the age of pubertal onset was no longer significant, suggesting the advancement of puberty was associated with increased BMI. So far, there is still inconsistency between the studies on impact of obesity on pubertal maturation in boys, although evidence that obesity might play a role in earlier onset of puberty is increasing.
A lot of large scaled epidemiologic studies indicate that secular trends of earlier pubertal maturation in girls are related with increased BMI or adiposity in the past 30 years. Activating effect of leptin on GnRH-gonadotropin axis is thought to be one of the possible mechanisms linking between body fat mass and earlier pubertal onset. Hyperinsulinemia in obesity may also contribute to earlier puberty by stimulation of androgen secretion, activation of aromatase in fat tissue and reduction of SHBG production from liver. Low birth weight and rapid catch up growth is known to be linked to hyperandrogenemia and subsequent early puberty. In contrast with girls, the data on the association of adiposity with pubertal onset in boys are much limited and, if any, the results are inconsistence. There are some limitations for studies in boys. Distinct pubertal milestones that can be easily obtained from an interview are not available, and pubertal staging by physical examination is more difficult in boys. Therefore, efforts are needed to overcome several limitations in future studies, such as objective pubertal staging, reliable measurement of body fat mass other than BMI, hormonal markers for pubertal development, and longitudinal study design to overcome genetic, nutritional and environmental variables.
Figures and Tables
Fig. 1
Effect of leptin as a permissive factor for pubertal onset. GnRH: gonadotropin releasing hormone, FSH: follicle stimulating hormone, LH: luteinizing hormone, PMV: ventral premammillary neurons.
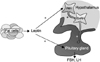
Fig. 2
Role of hyperinsulinemia in obesity on early pubertal maturation. SHBG: sex hormone binding globulin.
References
1. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003. 24:668–693.

2. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985. 107:317–329.

3. National Center for Health Statistics. Analytic and reporting guidelines: the third National Health and Nutrition Examination Survey, NHANES III (1988-94). 1997. Hyattsville: Center for Disease Control and Prevention.
4. Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009. 123:e932–e939.

5. Ma HM, Du ML, Luo XP, Chen SK, Liu L, Chen RM, et al. Pubertal Study Group of the Society of Pediatric Endocrinology and Genetic Disease, Chinese Medical Association. Onset of breast and pubic hair development and menses in urban chinese girls. Pediatrics. 2009. 124:e269–e277.

6. Park MJ, Lee IS, Shin EK, Joung H, Cho SI. The timing of sexual maturation and secular trends of menarchial age in Korean adolescents. Korean J Pediatr. 2006. 49:610–616.

7. Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997. 99:505–512.

8. Tsuzaki S, Matsuo N, Ogata T, Osano M. Lack of linkage between height and weight and age at menarche during the secular shift in growth of Japanese children. Ann Hum Biol. 1989. 16:429–436.

9. de Muinich Keizer SM, Mul D. Trends in pubertal development in Europe. Hum Reprod Update. 2001. 7:287–291.

10. Mul D, Fredriks AM, van Buuren S, Oostdijk W, Verloove-Vanhorick SP, Wit JM. Pubertal development in The Netherlands 1965-1997. Pediatr Res. 2001. 50:479–486.

11. Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988-1994. Arch Pediatr Adolesc Med. 2001. 155:1022–1028.
12. Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010. 95:263–270.

13. Song JE, Yi YY, Hwang IT, Lee HR, Lim JS, Yang S. Testicular volume in Korean boys. J Korean Soc Pediatr Endocrinol. 2010. 15:14–18.
14. Ma HM, Chen SK, Chen RM, Zhu C, Xiong F, Li T, et al. Pubertal Study Group of the Society of Pediatric Endocrinology and Genetic Disease, Chinese Medical Association. Pubertal development timing in urban Chinese boys. Int J Androl. 2011. 34:e435–e445.

15. Ogden C, Carroll M. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2007-2008. Retrieved from National center for health statistics, Center for Disease Control and Prevention. Last accessed on 30 July 2012. Available from:
http://www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.pdf.
16. Chinn S, Rona RJ. Prevalence and trends in overweight and obesity in three cross sectional studies of British Children, 1974-94. BMJ. 2001. 322:24–26.

17. Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006. 1:11–25.

18. Magarey AM, Daniels LA, Boulton TJ. Prevalence of overweight and obesity in Australian children and adolescents: reassessment of 1985 and 1995 data against new standard international definitions. Med J Aust. 2001. 174:561–564.

19. Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002. 75:971–977.

20. Murata M. Secular trends in growth and changes in eating patterns of Japanese children. Am J Clin Nutr. 2000. 72:5 Suppl. 1379S–1383S.

21. Chu MA, Choe BH. Obesity and metabolic syndrome among children and adolescents in Korea. J Korean Med Assoc. 2010. 53:142–152.

22. Moon JS. Secular trends of body sizes in Korean children and adolescents: from 1965 to 2010. Korean J Pediatr. 2011. 54:436–442.

23. Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001. 108:347–353.

24. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002. 110:903–910.

25. Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002. 110:e43.

26. Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child. 1971. 46:695–701.

27. Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002. 57:Suppl 2. 2–14.

28. Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978. 14:141–148.

29. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996. 12:318–320.

30. Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998. 392:398–401.

31. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998. 18:213–215.

32. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999. 341:879–884.

33. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004. 351:987–997.

34. Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997. 138:855–858.

35. Cheung CC, Thornton JE, Nurani SD, Clifton DK, Steiner RA. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology. 2001. 74:12–21.

36. Clayton PE, Gill MS, Hall CM, Tillmann V, Whatmore AJ, Price DA. Serum leptin through childhood and adolescence. Clin Endocrinol (Oxf). 1997. 46:727–733.

37. Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999. 84:899–905.

38. Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999. 84:2903–2911.

39. Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997. 94:1023–1028.
40. Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011. 121:355–368.

41. Dennis MS, Melvin MG. Shlomo M, Kenneth SP, Larsen PR, Kronenberg HM, editors. Chapter 25. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. Williams textbook of endocrinology. 2011. 12th ed. Philadelphia: WB Saunders Co.;1087–1104.
42. Moran A, Jacobs DR Jr, Steinberger J, Cohen P, Hong CP, Prineas R, et al. Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab. 2002. 87:4817–4820.

43. Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002. 26:701–709.

45. Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth--a review. Int J Obes Relat Metab Disord. 2003. 27:869–874.

46. Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006. 95:904–908.

47. Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche--normal variant or forerunner of adult disease? Endocr Rev. 2000. 21:671–696.

48. Ong KK, Potau N, Petry CJ, Jones R, Ness AR, Honour JW, et al. Avon Longitudinal Study of Parents and Children Study Team. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab. 2004. 89:2647–2651.

49. Ibáñez L, Jiménez R, de Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics. 2006. 117:117–121.

50. Ibáñez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab. 2006. 91:2888–2891.

51. Holly JM, Smith CP, Dunger DB, Howell RJ, Chard T, Perry LA, et al. Relationship between the pubertal fall in sex hormone binding globulin and insulin-like growth factor binding protein-I. A synchronized approach to pubertal development? Clin Endocrinol (Oxf). 1989. 31:277–284.

52. Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999. 20:535–582.

53. Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009. 20:237–242.

54. Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, McClanahan BS. Early sexual maturation, body composition, and obesity in African-American girls. Obes Res. 2004. 12:Suppl. 64S–72S.

55. Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003. 111:844–850.

56. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009. 123:84–88.

57. Mamun AA, Hayatbakhsh MR, O'Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation--pathways of association with young adults' overweight: a longitudinal study. Int J Obes (Lond). 2009. 33:14–20.

58. Buyken AE, Karaolis-Danckert N, Remer T. Association of prepubertal body composition in healthy girls and boys with the timing of early and late pubertal markers. Am J Clin Nutr. 2009. 89:221–230.

59. Boyne MS, Thame M, Osmond C, Fraser RA, Gabay L, Reid M, et al. Growth, body composition, and the onset of puberty: longitudinal observations in Afro-Caribbean children. J Clin Endocrinol Metab. 2010. 95:3194–3200.

60. Yoon JR, Ahn JH, Huh K, Park MJ. Body composition in girls with precocious puberty. Korean J Obes. 2010. 19:95–100.
61. Vignolo M, Naselli A, Di Battista E, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr. 1988. 147:242–244.

62. Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc Med. 2010. 164:139–144.

63. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010. 140:399–410.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download