INTRODUCTION
The demand for colonoscopy, which has been recognized as a pivotal tool in colorectal cancer (CRC) screening, has increased significantly in many developed countries.
12 The importance of colonoscopy is related to the detection and removal of colorectal adenomatous polyps during the procedure, which may prevent the development of CRC and ultimately lead to a significant reduction in mortality.
3 However, many studies have revealed that colonoscopy is not fully effective as a method of protecting the colon from CRC, particularly interval cancer.
4567 Interval cancer development has been suggested to correlate with the adenoma detection rate, an indicator of colonoscopy quality that varies among physicians; specifically, endoscopists with lower adenoma detection rates are associated with a higher risk of interval cancer.
89 Therefore, increasing attention has been paid to colonoscopy quality, and specific recommendations of quality indicators have been made to ensure the continuous improvement of colonoscopy quality and effectiveness.
10
Optimal reporting of colonoscopic procedures should be required in order to measure the performance of colonoscopists and monitor quality indicators in clinical practice. In 2007, a standardized colonoscopy reporting system was developed by the Quality Assurance Task Force of the National Colorectal Cancer Roundtable in an effort to facilitate continuous quality improvements across diverse practices using colonoscopy.
11 Nevertheless, the level of reporting of these indicators has often been insufficient in daily clinical practice.
121314
South Korea implemented the National Cancer Screening Program in 1999 to address the high incidence of cancer; as a result, a tremendous number of colonoscopies were conducted in clinical practice.
15 Nevertheless, no data have demonstrated the actual situation of colonoscopy reporting systems in daily clinical practices in South Korea, although these data might provide a comprehensive insight into colonoscopy quality in this region.
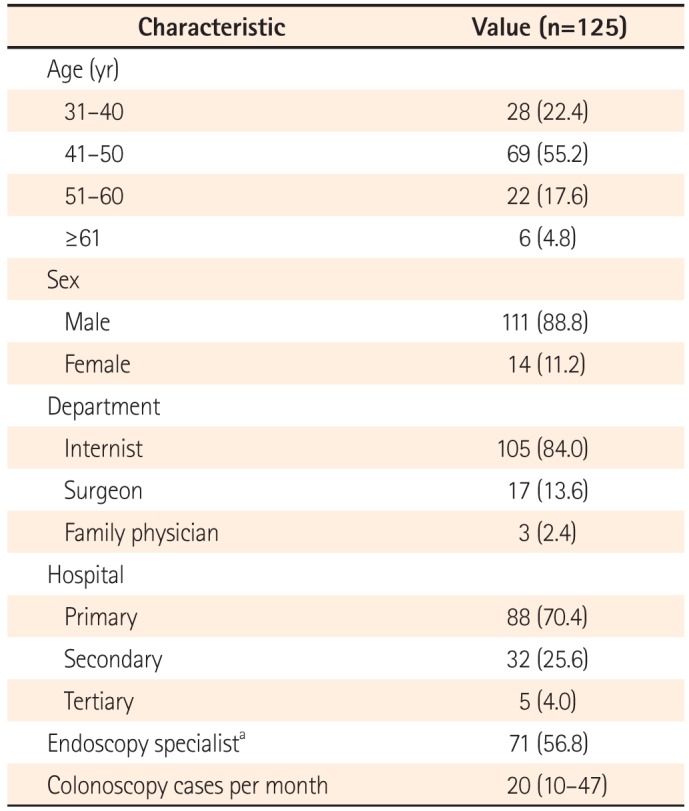
The present study aimed to assess the quality of colonoscopy reports using a standardized questionnaire administered to a wide range of clinical centers with different expertise levels and workloads located in the Daegu-Gyeongbuk province in southeastern South Korea. The study also evaluated predictive factors of a failure to use colonoscopy reporting systems.
DISCUSSION
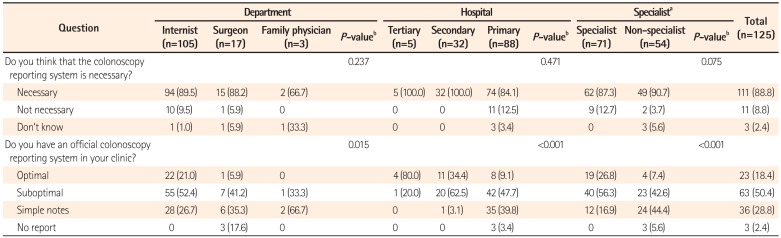
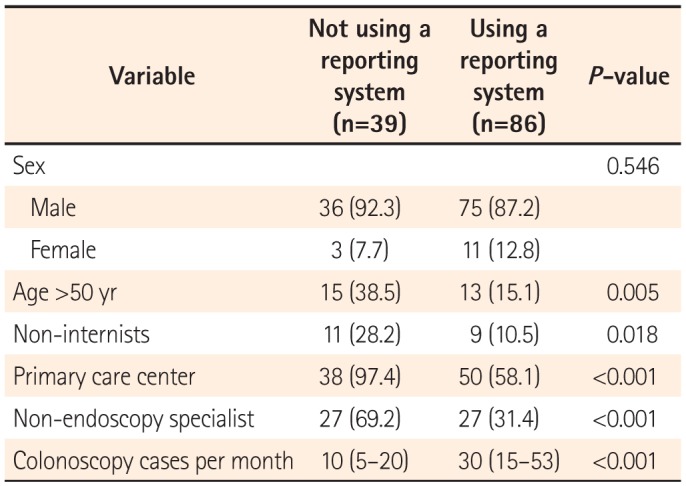
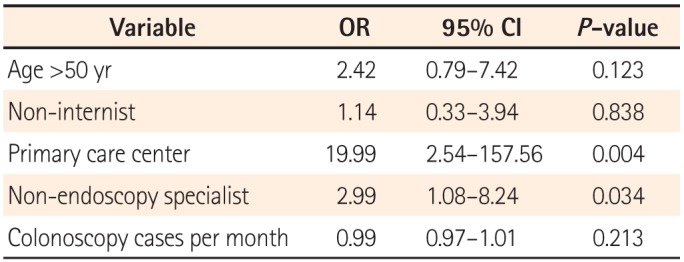
The results of the present questionnaire-based study revealed broad variability in the quality of colonoscopy reporting systems among physicians, thus reflecting the real-world experience in a southeastern area of South Korea. We found that the reporting system quality was considerably inadequate, although this depended on the department, hospital care level, and endoscopy specialist accreditation status. One-third of the responders did not use a colonoscopy reporting system for various reasons, and a non-endoscopy specialist status and working in a primary clinic were factors significantly associated with this outcome.
In general, endoscopy reports are considered fundamental elements of medical records because they serve as an important means of communicating information related to endoscopic findings, procedures, and recommendations among physicians. In addition, colonoscopy reporting has received significantly increased attention, as a recent body of evidence emphasized the significance of colonoscopy quality issues with regard to CRC screening.
10 Many colonoscopy quality indicators, including bowel preparation, withdrawal time, and adenoma detection rate, are only meaningful when they are adequately recorded. Colonoscopy quality cannot not be determined without the accurate and complete reporting of these colonoscopy parameters. In this context, the results of the present study raised profound concerns about the quality of colonoscopies performed in this region.
Although the exact impact of incomplete colonoscopy reporting is not clear, we can surmise several plausible consequences. For instance, it is impossible to recommend an optimal follow-up schedule of procedures without knowing the bowel preparation status.
13
Detailed information about polyps is essential in clinical practice, particularly with regard to communication between referring physicians and physicians at referral centers; for example, polyp size and morphology data could greatly facilitate risk assessments of the malignant potential of tumors and decisions regarding optimal treatment approaches.
1718 Without this information, unnecessary repeat colonoscopies may be performed, with consequent increased costs and greater procedural risks. Most of all, the greatest concern associated with insufficient colonoscopy documentation might be the development of interval cancer, although no evidence has suggested that poor colonoscopy reporting is directly related to the risk of interval cancer. Further studies of the precise impacts of insufficient colonoscopy reporting on clinical outcomes are needed. At the very least, we can measure and improve colonoscopists' performances to determine whether the reporting is good.
One notable finding of our study was the existence of different grades of reporting quality according to the hospital, department, procedure number, and endoscopy specialist accreditation. Furthermore, we found that working at a primary care center and a non-endoscopy specialist status were independent predictors of failure to use a colonoscopy reporting system. This result was in agreement with the findings of a previous study, in which gastroenterologists were found to achieve better reporting and performance quality, compared with non-gastroenterologists.
19 Indeed, evidence has shown an association between colonoscopy performed by non-gastroenterologists and an increased risk of interval cancer.
202122 Although it is difficult to directly compare studies from different countries because of variations in medical training systems, South Korean endoscopy specialists might correspond to gastroenterologists in previous studies, as both certifications require a certain period of specified training and examination to ensure good-quality endoscopic examinations.
1621 Another South Korean study also reported that the achievement of endoscopy specialty qualification by the KSGE was associated with high-quality endoscopy performance.
16
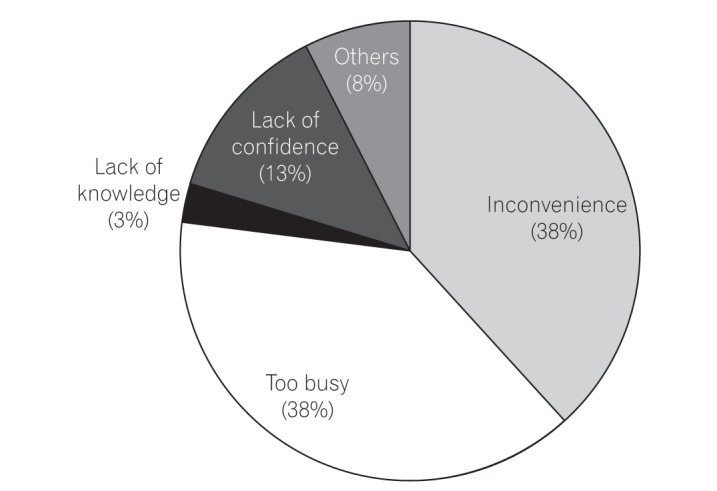
As indicated in our study, which identified "inconvenience" or being "too busy" as the main reasons for not reporting colonoscopy parameters, the main obstacle to complete colonoscopy reporting might be the physician's perception of colonoscopy reporting as a slow, laborious task. For clinical and quality purposes, additional time and effort are needed when inputting data into colonoscopy reports.
23 For instance, although the KSGE has begun to provide and encourage the use of a standardized colonoscopy report form on its website,
24 this form might not be useful unless it is incorporated into the medical records systems of individual centers. One potential solution for improved compliance would be a computerized endoscopy reporting system with a structured input matrix, instead of a handwritten or free-text dictation system.
25
In the same context, we noticed a significant discrepancy between the perceived necessity of colonoscopy reporting and implementation of this necessity in actual clinical practices. Although most responders understood that colonoscopy reporting is essential, they ignored this knowledge in their routine clinical practices. Therefore, efforts should be undertaken to develop and provide computerized colonoscopy reporting systems that do not require dual data entry and can be easily utilized in a primary clinical setting.
The strength of this study was its evaluation of the situation of real-life colonoscopy documentation across a broad spectrum of physicians; accordingly, our results offer a comprehensive insight into colonoscopy quality in this region. Because most studies of colonoscopy quality have been conducted in non-daily clinical settings such as screening programs and research-based academic hospitals, very limited data on primary care centers are available. To our knowledge, this study was the first to evaluate colonoscopists' opinions and the actual situation of colonoscopy reporting systems in daily clinical practices in Asia.
However, this study had some limitations. First, we did not evaluate specific parameters, such as the cecal intubation rate, withdrawal time, and adenoma detection rate, which are known surrogate markers of colonoscopy quality. Therefore, the performance quality achieved by individual colonoscopists remains unknown. Second, because this study featured a cross-sectional design, we could not evaluate changes in the quality of reporting over time or observe the effects of interventions on the quality of reporting in this population.
In conclusion, this questionnaire-based study observed a wide variation in the quality of colonoscopy reporting systems that depended on the hospital care level and endoscopist's expertise, indicating much room for improvement in colonoscopy quality control. In particular, there is an urgent need for the development of endoscopist-friendly, computerized colonoscopy reporting systems that do not require dual data entry in daily clinical practices in South Korea. Further study is needed to confirm the precise impact of inadequate-quality colonoscopy reporting systems on clinical outcomes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download