Abstract
The treatment of constipation aims to regulate the frequency and quantity of stool in order to promote successful defecation. Numerous studies on pharmacologic treatments and non-pharmacologic therapies for constipation have attempted to overcome limitations such as temporary and insufficient efficacy. Conventional laxatives have less adverse effects and are inexpensive, but often have limited efficacy. Recently developed enterokinetic agents and intestinal secretagogues have received attention owing to their high efficacies and low incidences of adverse events. Studies on biofeedback and surgical treatment have focused on improving symptoms as well as quality of life for patients with refractory constipation.
Successful defecation requires sufficient luminal quantity of stool, adequate colonic contractility to evacuate stool toward the anus, and coordinated anorectal movements. Lifestyle modifications such as high-fiber diet and the use of conventional laxatives, as a first step for the treatment of constipation, are not effective in many cases.12 For patients with more troublesome constipation, the physiological functions of the colon and pelvic floor are evaluated to identify the underlying causes. These patients are then treated by the administration of drugs with novel mechanisms, behavioral treatments, or surgical intervention. Numerous studies have been conducted on constipation thus far to overcome the limitations of current treatments. This article aims to identify recent trends in the treatment of constipation.
Polyethylene glycol (PEG) is an osmotic laxative that is not absorbed by the small intestine, and maintains high osmotic pressure in the colon. Thus, these hypertonic products exert its effects by driving water into the lumen. Well-designed studies on chronic constipation (CC) have shown that PEG is effective in improving stool frequency, consistency, and straining during defecation.345 In a randomized controlled trial, PEG was administered to patients with CC for 6 months and no serious adverse events were observed.6 In a recent study of female patients with CC, PEG was shown to possess comparable efficacy to and better tolerability than prucalopride.7 Furthermore, PEG alleviated constipation symptoms in patients with IBS with constipation (IBS-C).8 PEG has fewer adverse effects and is highly cost-effective, and is thus valued as a first-line drug for constipation.
Bisacodyl is a polyphenolic stimulant laxative, and is assumed to inhibit the absorption of water and electrolytes in the colon and promote bowel movement by stimulating the colonic myenteric plexus. It is used for short-term bowel preparation in patients with CC.910 Bisacodyl has been reported to improve stool frequency and consistency significantly in patients with CC compared to placebo, with adverse events similar to those of the placebo.11 In a randomized, double-blind, placebo-controlled trial conducted in the UK, bisacodyl increased the number of complete spontaneous bowel movements (CSBMs) per week, improved quality of life, and was well tolerated.12 This drug is recommended as a first-line treatment for CC owing to its cost-effectiveness and relatively high efficacy.13 However, further research on its long-term efficacy and adverse events is required.
Tegaserod is a partial 5-hydroxytryptamine type 4 (5-HT4) receptor agonist and effective in treating female patients with CC as well as IBS-C. In a large-scale study of patients with CC, tegaserod increased CSBMs, improved constipation-related symptoms and patient satisfaction, and was effective for 12 weeks.14 The US Food and Drug Administration (FDA) regulated the general use of tegaserod in 2007 owing to the risk of serious cardiovascular side effects. Its use is currently restricted, under an investigational new drug protocol, to women below 55 years of age. However, recent multicenter studies conducted in the US and Europe showed that tegaserod has high efficacy in female patients who had IBS with mixed bowel habits or IBS-C.15 In a meta-analysis of 11 randomized controlled studies, the comparative efficacy of tegaserod was reported to be 0.85 (95% CI, 0.80-0.90), suggesting that it is capable of ameliorating symptoms.16 In a study of 81 Korean women with IBS-C, tegaserod was administered for 4 weeks and improved both symptoms and quality of life.17 A 6-month observational cohort study conducted in the US reported that there was no difference in the risk of cardiovascular events between the tegaserod group, consisting of 52,229 subjects, and the control group.18 Based on these findings, some researchers argue against the FDA's decision to discontinue the marketing of tegaserod. However, the risks associated with the use of tegaserod are still considered to be higher than its benefits,1920 and no new research on tegaserod has been reported since the recent development of new drugs with improved cardiovascular safety.
Prucalopride is a drug that acts as a selective, high-affinity 5-HT4 receptor full agonist, and is effective in treating constipation by increasing colonic contractility. In three pivotal trials, prucalopride was verified to be effective in increasing CSBMs per week, and improving perceived disease severity and quality of life in patients with CC.212223 In a study of 620 patients with CC, participants receiving 2 or 4 mg of prucalopride for 12 weeks had increased one or more CSBMs per week compared to patients in the control group.21 In another trial of 713 patients with CC, administration of 2 or 4 mg of prucalopride increased frequency of three or more CSBMs per week, and improved evacuation completeness, perceived disease severity, and quality of life.22 In a study of elderly patients aged 65 years and older with CC, 1 mg of prucalopride was administered for 4 weeks, and no changes in electrocardiogram or corrected QT (QTc) interval were reported, indicating its safety for the treatment of CC in the elderly.2425 A study of Asian subjects with CC reported its high efficacy and safety similar to the results from clinical trials with Western populations.26 In a pooled analysis of the study with Asian subjects and the three pivotal trials, increased stool frequency with an average of three or more CSBMs per week was demonstrated in Asian (34% vs. 11%, P<0.001) and non-Asian women (24.6% vs. 10.6%, P<0.001), and prucalopride was found to be safe and well-tolerated.27 It was also effective in improving the abdominal symptoms of CC such as abdominal discomfort, bloating, straining, and painful bowel movements.28 In a study of a small number of patients, prucalopride was shown to be effective not only in the treatment of slow transit constipation, but also obstructed defecation and IBS-C.29 Common adverse events included diarrhea and headache. No effects on human ether-a-go-go-related gene (hERG) potassium channels, QTc interval, or cardiovascular adverse events were reported.212223 The European Medicines Agency (EMA) approved its use in female patients with CC. However, further research is required to verify the long-term effects, and effects on other types of constipation such as IBS-C.
Lubiprostone is a prostaglandin E1 derivative that activates type-2 chloride channels (ClC-2) on the apical surface of epithelial cells lining the gastrointestinal tract, and triggers the secretion of water-containing electrolytes. It then enters the intestines and induces bowel movements. Lubiprostone was approved for use by the FDA in adults with CC in 2006, and in female patients with IBS-C aged 18 years and above in 2008. In studies published after approval, lubiprostone was shown to significantly improve the symptoms of patients with IBS-C.3233 However, in a long-term follow-up study of 248 patients with CC, only 51% of patients were reported to have completed 48 weeks of drug administration, despite the fact that dose moderation was allowed based upon the severity of adverse events. The most common treatment-related adverse events were nausea (19.8%), diarrhea (9.7%), abdominal distension (6.9%), and headache (6.9%).34 Recent studies on lubiprostone have focused on its effects on intestinal secretion, mucus-mobilization, and microbiota.3536
Guanylate cyclase-C is a receptor in the intestinal mucosal cells that activates the cystic fibrosis transmembrane conductance regulator (CFTR). Through activation of the CFTR chloride channel, it promotes intestinal secretion of water and electrolytes. Linaclotide, a guanylate cyclase-C agonist, was administered for 5 days in a study of 36 females with IBS-C, and resulted in accelerated transit time in the ascending colon.37 In two phase III studies of 1,604 patients with IBS-C, linaclotide significantly alleviated symptoms such as abdominal pain, discomfort, bloating, and excessive straining, and significantly increased stool frequency.3839 The improvements in abdominal pain or discomfort were significantly different between the treatment group (290 µg per day) and the placebo group. These groups had 54.8% vs. 41.8% improvement after 12 weeks of administration, and 53.6% vs. 36.0% improvement after 26 weeks of administration.40 Linaclotide affects the colonic sensory afferents in patients with chronic visceral hypersensitivity by reducing the activity of pain-sensing fibers by elevating extracellular cyclic guanosine-3',5'-monophosphate (cGMP) levels.41 The superior effect of linaclotide in patients with CC was verified in a randomized, multicenter, double-blind study of 1,276 patients with CC.42 In addition, improvements in abdominal and bowel symptoms were reported in patients with CC.43 Linaclotide was approved by the FDA in 2012 for the treatment of IBS-C and CC. However, further research of its long-term efficacy and safety is needed.
Studies on the effect of probiotics for the treatment of CC are heterogeneous in their designs, and very few are well designed. In a systematic review of five randomized controlled trials, Bifidobacterium lactis DN-173 010, Lactobacillus casei Shirota, and Escherichia coli Nissle 1917 were reported to improve defecation frequency and stool consistency in patients with CC.44 A recent randomized, double-blind, placebo-controlled trial in patients with CC reported the efficacy of a microbial cell preparation containing fructooligosaccharide, Bifidobacterium, and Lactobacillus.45 However, this effect could not be directly correlated with the effectiveness of probiotics, since probiotics were administered in a form of synbiotics. In a 2-week VSL#3 study in patients with CC, clinical symptoms improved and fewer Bifidobacterium and bacteroides species were detected in the feces of patients with constipation.46 However, the mechanism by which probiotics contribute to the treatment of patients with CC has not been identified, and long-term follow-up studies are rare. Therefore, it is difficult to make conclusions about the efficacy of probiotics in the treatment of CC.
Several studies have reported an association between the overgrowth of methane-producing intestinal microbiota and constipation. Overgrowth of methane-producing intestinal microbes has been reported to have a higher correlation with CC than IBS-C.47 In a recent study reporting an association between CC and the presence of methanogenic flora, a glucose breath test was performed on 96 patients with CC and 106 healthy subjects. Based on ≥3 parts per million as a positive baseline value, the positivity in the control group, normal transit constipation group, and slow transit constipation group were 28%, 44%, and 75%, respectively.48 Sixty-two patients with constipation and 49 healthy subjects were enrolled in a colonic transit study using a radio-opaque marker and a lactulose breath test. The results showed that positivity in the control group, normal-transit constipation group, and slow-transit constipation group were 12%, 13%, and 59%, respectively.49 Positive effects of neomycin and rifaximin treatment on patients with IBS-C have been reported.5051 In a recent small-scale study of patients with methane-positive IBS-C, a combination of neomycin and rifaximin was found to be more effective than neomycin alone.52 However, the criteria for the breath test have not been properly established, and it is therefore difficult to conclude that there is an association between methane production and constipation. Prior to commencing treatment with antibiotics, standardization of diagnostic methods, identification of mechanisms, evaluation of antibiotic-resistant bacteria in long-term use, and safety should be established.
Normally, more than 95% of bile acid is reabsorbed in the terminal ileum. Unabsorbed bile acid moves to the colon to promote peristalsis and activates adenylate cyclase, which increases permeability of the mucous membrane of the colon, resulting in diarrhea.53 Elobixibat, a selective ileal bile acid transporter inhibitor administered at a dose of 20 mg once daily for 2 weeks to 36 female patients with CC, reduced colonic transit and improved symptoms such as stool consistency, stool frequency, and excessive straining.54 In a phase IIb trial of 190 patients with CC, patients were randomly assigned to receive either placebo or elobixibat 5 mg, 10 mg, or 15 mg once daily for 8 weeks. After the first week, the 10 mg and 15 mg groups showed signs of improvement in stool frequency and constipation symptoms compared to the control group, and this effect was maintained for the entire 8-week period. The most common adverse events included abdominal pain and diarrhea, which were dose-dependent.55 However, more research on the efficacy, safety, and the effect of excessive bile acid on the mucous membrane of the colon is required.
Opioids exert an analgesic effect by binding to µ-opioid receptors in the central nervous system. They can cause constipation by inhibiting bowel movements through the µ-opioid receptors in the gastrointestinal tract. In a study of patients with opioid-induced bowel dysfunction, alvimopan, a peripherally acting µ-opioid receptor antagonist (PAMORA), significantly improved stool frequency during the 8 hours and the time required for the first evacuation compared to placebo.56 Alvimopan is reportedly effective in patients with postoperative ileus and bowel resection who received patient-controlled analgesia.57 Alvimopan was approved by the FDA in 2008. Methylnaltrexone, approved by the FDA in 2008, is a selective inhibitor of opioid receptors located in the intestinal muscle cells, and normalizes bowel function without affecting the analgesic effects of opioids.58 Preliminary studies on TD-1211 and NKTR-118, new oral PAMORAs, have been reported.59 Lubiprostone, a selective ClC-2 channel activator approved by the FDA in 2013, was administered at 48 µg per day for 12 weeks to patients with opioid-induced constipation, spontaneous stool frequency, abdominal discomfort, excessive straining, and stool consistency improved to a greater extent in the lubiprostone than that in the placebo group.60 In addition, naltrexone extended-release, a combination of naltrexone and morphine, and tapentadol, a combination of a norepinephrine reuptake inhibitor and a µ-opioid receptor agonist, are under development.61 These medications are expected to have more significance in the future owing to the increased use of opioids.
Biofeedback is a learning method using electric or mechanical devices to improve patients' control over their own biological responses through trial and error, and to increase their recognition of biological responses. In defecatory disorders, biofeedback is mainly used for improving coordination or recovering the contractibility of the pelvic floor muscles. Biofeedback has an approximately 70% success rate in many studies. Some studies were not well designed, and caution is therefore required.6263 In a 5-week trial of patients with pelvic floor dyssynergia, patients were randomly assigned to the PEG or biofeedback groups, and improvement in constipation was evaluated 1 and 2 years after treatment. Short-term results showed that PEG and biofeedback had 20% and 80% efficacy, respectively, and that the effect was maintained for 2 years.64 In another study, the effectiveness of biofeedback therapy was reportedly maintained for over a year.65 However, a retrospective study that examined the completion rate and efficacy of biofeedback therapy reported that only 48% of patients with dyssynergic defecation and 44% of patients with fecal incontinence completed the biofeedback therapy. In terms of the efficacy of short-term therapy, 60% of patients with dyssynergic defecation and 80% of patients with fecal incontinence responded that it was effective.66 These results indicate the necessity for further research on the efficacy and methodology of biofeedback in clinical practice.
Sacral nerve stimulation can improve the symptoms of constipation by stimulating parasympathetic sacral nerves in the intestinal tract, which play an important role in accelerating movement of the colon. There have been anecdotal reports of sacral nerve stimulation for patients with slow transit constipation who do not respond to conventional treatment.67 In a study of 45 patients with refractory constipation, 39 (87%) patients showed improvements in their symptoms.68 However, in another study, 58% of patients experienced pain or lack of efficacy.69 Moreover, after three years of observation following definitive sacral nerve stimulation treatment, the slow transit constipation group showed poor long-term efficacy.70 Although sacral nerve stimulation therapy may be considered as a non-invasive method for treating patients with intractable constipation in the future, it lacks sufficient foundation to act as a first-line treatment for refractory constipation, and further research on appropriate stimulation methods is required.
Total or subtotal colectomy may be considered for patients with intractable constipation who do not respond to medical treatment. Post-operative patient satisfaction varies, ranging from 39-100%.71 In a study of a relatively large number of patients using a validated questionnaire, colectomy and ileorectal anastomosis were shown to improve long-term quality of life.72 Patients with no abnormalities in the small bowel manometry before surgery tend to have good prognosis.73 Therefore, patients should be carefully screened prior to surgery through detailed examination of their intestinal motility.
Current treatments for constipation include pharmacologic agents such as laxatives and non-pharmacologic therapies such as biofeedback, sacral nerve stimulation, and surgery. Newly developed serotonergic agents have overcome limitations, and have shown superior clinical results. In addition, a guanylate cyclase-C agonist has been reported to improve the symptoms of constipation, and its high efficacy has been verified. Other new agents under evaluation include the bile acid transporter inhibitors, antibiotics, and probiotics (Table 1). Non-pharmacologic therapies have been attempted for treating defecatory disorders and intractable constipation.
References
1. Wald A, Scarpignato C, Mueller-Lissner S, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther. 2008; 28:917–930. PMID: 18644012.

2. Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002; 97:1986–1993. PMID: 12190165.
3. Andorsky RI, Goldner F. Colonic lavage solution (polyethylene glycol electrolyte lavage solution) as a treatment for chronic constipation: a double-blind, placebo-controlled study. Am J Gastroenterol. 1990; 85:261–265. PMID: 2178398.
4. Attar A, Lémann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999; 44:226–230. PMID: 9895382.
5. DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MB. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000; 95:446–450. PMID: 10685748.
6. Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007; 102:1436–1441. PMID: 17403074.
7. Cinca R, Chera D, Gruss HJ, Halphen M. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation - a comparison in a controlled environment. Aliment Pharmacol Ther. 2013; 37:876–886. PMID: 23480216.
8. Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol. 2013; 108:1508–1515. PMID: 23835436.
9. Delegge M, Kaplan R. Efficacy of bowel preparation with the use of a prepackaged, low fibre diet with a low sodium, magnesium citrate cathartic vs. a clear liquid diet with a standard sodium phosphate cathartic. Aliment Pharmacol Ther. 2005; 21:1491–1495. PMID: 15948817.
10. Verghese VJ, Ayub K, Qureshi W, Taupo T, Graham DY. Low-salt bowel cleansing preparation (LoSo Prep) as preparation for colonoscopy: a pilot study. Aliment Pharmacol Ther. 2002; 16:1327–1331. PMID: 12144583.
11. Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2006; 23:1479–1488. PMID: 16669963.
12. Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 2011; 9:577–583. PMID: 21440672.
13. American Gastroenterological Association. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013; 144:211–217. PMID: 23261064.
14. Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005; 100:362–372. PMID: 15667494.
15. Chey WD, Paré P, Viegas A, Ligozio G, Shetzline MA. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008; 103:1217–1225. PMID: 18477346.
16. Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009; 104:1831–1843. PMID: 19471254.
17. Kim YS, Choi SC, Park JM, et al. The effect of tegaserod on symptoms and quality of life in Korean women with irritable bowel syndrome with constipation. J Neurogastroenterol Motil. 2010; 16:61–70. PMID: 20535328.
18. Loughlin J, Quinn S, Rivero E, et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study. J Cardiovasc Pharmacol Ther. 2010; 15:151–157. PMID: 20200325.
19. Brandt LJ. The FDA's decision-making process: isn't it time to temper the principle of protective paternalism? Am J Gastroenterol. 2008; 103:1226–1227. PMID: 18477347.
20. Wood P. Tegaserod in the treatment of constipation-predominant irritable bowel syndrome. Do the risks outweigh the benefits? Naunyn Schmiedebergs Arch Pharmacol. 2012; 385:1–3. PMID: 21969099.
21. Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008; 358:2344–2354. PMID: 18509121.
22. Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009; 58:357–365. PMID: 18987031.
23. Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation - a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009; 29:315–328. PMID: 19035970.
24. Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009; 21:1256–e117. [1264]. PMID: 19751247.
25. Müller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol Motil. 2010; 22:991–998. PMID: 20529205.
26. Ke M, Zou D, Yuan Y, et al. Prucalopride in the treatment of chronic constipation in patients from the Asia-Pacific region: a randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. 2012; 24:999–e541. [1009]. PMID: 22882724.
27. Ke M, Tack J, Quigley EM, et al. Effect of prucalopride in the treatment of chronic constipation in Asian and non-Asian women: a pooled analysis of 4 randomized, placebo-controlled studies. J Neurogastroenterol Motil. 2014; 20:458–468. PMID: 25273116.
28. Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014; 26:21–27. PMID: 24106924.
29. Jadav AM, McMullin CM, Smith J, Chapple K, Brown SR. The association between prucalopride efficacy and constipation type. Tech Coloproctol. 2013; 17:555–559. PMID: 23703575.
30. Goldberg M, Li YP, Johanson JF, et al. Clinical trial: the efficacy and tolerability of velusetrag, a selective 5-HT4 agonist with high intrinsic activity, in chronic idiopathic constipation - a 4-week, randomized, double-blind, placebo-controlled, dose-response study. Aliment Pharmacol Ther. 2010; 32:1102–1112. PMID: 21039672.
31. Mozaffari S, Didari T, Nikfar S, Abdollahi M. Phase II drugs under clinical investigation for the treatment of chronic constipation. Expert Opin Investig Drugs. 2014; 23:1485–1497.
32. Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008; 27:685–696. PMID: 18248656.
33. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome - results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009; 29:329–341. PMID: 19006537.
34. Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB Jr, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci. 2011; 56:2639–2645. PMID: 21769655.
35. Musch MW, Wang Y, Claud EC, Chang EB. Lubiprostone decreases mouse colonic inner mucus layer thickness and alters intestinal microbiota. Dig Dis Sci. 2013; 58:668–677. PMID: 23329012.
36. Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci. 2012; 57:2826–2845. PMID: 22923315.
37. Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007; 133:761–768. PMID: 17854590.
38. Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012; 107:1714–1724. PMID: 22986440.
39. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012; 107:1702–1712. PMID: 22986437.
40. Quigley EM, Tack J, Chey WD, et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther. 2013; 37:49–61. PMID: 23116208.
41. Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology. 2013; 145:1334–1346. PMID: 23958540.
42. Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011; 365:527–536. PMID: 21830967.
43. Chang L, Lembo AJ, Lavins BJ, et al. The impact of abdominal pain on global measures in patients with chronic idiopathic constipation, before and after treatment with linaclotide: a pooled analysis of two randomised, double-blind, placebo-controlled, phase 3 trials. Aliment Pharmacol Ther. 2014; 40:1302–1312. PMID: 25312449.
44. Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010; 16:69–75. PMID: 20039451.
45. Jayasimhan S, Yap NY, Roest Y, Rajandram R, Chin KF. Efficacy of microbial cell preparation in improving chronic constipation: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2013; 32:928–934. PMID: 23561636.
46. Kim SE, Choi SC, Park KS, et al. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J Neurogastroenterol Motil. 2015; 21:111–120. PMID: 25537674.
47. Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014; 20:31–40. PMID: 24466443.
48. Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010; 105:1407–1411. PMID: 19953090.
49. Lee KM, Paik CN, Chung WC, Yang JM, Choi MG. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol. 2013; 25:726–732. PMID: 23395994.
50. Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol. 2010; 44:547–550. PMID: 19996983.
51. Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006; 51:1297–1301. PMID: 16832617.
52. Pimentel M, Chang C, Chua KS, et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig Dis Sci. 2014; 59:1278–1285. PMID: 24788320.
53. Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999; 159:2647–2658. PMID: 10597755.
54. Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011; 106:2154–2164. PMID: 21876564.
55. Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of A3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011; 106:1803–1812. PMID: 21606974.
56. Paulson DM, Kennedy DT, Donovick RA, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction-a 21-day treatment-randomized clinical trial. J Pain. 2005; 6:184–192. PMID: 15772912.
57. Büchler MW, Seiler CM, Monson JR, et al. Clinical trial: alvimopan for the management of post-operative ileus after abdominal surgery: results of an international randomized, double-blind, multicentre, placebo-controlled clinical study. Aliment Pharmacol Ther. 2008; 28:312–325. PMID: 19086236.
58. Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008; 358:2332–2343. PMID: 18509120.
59. Diego L, Atayee R, Helmons P, Hsiao G, von Gunten CF. Novel opioid antagonists for opioid-induced bowel dysfunction. Expert Opin Investig Drugs. 2011; 20:1047–1056.
60. Cryer B, Katz S, Vallejo R, Popescu A, Ueno R. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2014; 15:1825–1834. PMID: 24716835.
61. Camilleri M. Pharmacological agents currently in clinical trials for disorders in neurogastroenterology. J Clin Invest. 2013; 123:4111–4120. PMID: 24084743.
62. Heymen S, Jones KR, Scarlett Y, Whitehead WE. Biofeedback treatment of constipation: a critical review. Dis Colon Rectum. 2003; 46:1208–1217. PMID: 12972965.
63. Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev. 2014; 3:CD008486. PMID: 24668156.
64. Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006; 130:657–664. PMID: 16530506.
65. Lee BH, Kim N, Kang SB, et al. The Long-term clinical efficacy of biofeedback therapy for patients with constipation or fecal incontinence. J Neurogastroenterol Motil. 2010; 16:177–185. PMID: 20535349.
66. Jodorkovsky D, Dunbar KB, Gearhart SL, Stein EM, Clarke JO. Biofeedback therapy for defecatory dysfunction: "real life" experience. J Clin Gastroenterol. 2013; 47:252–255. PMID: 23328298.
67. Vitton V, Roman S, Damon H, Barth X, Mion F. Sacral nerve stimulation and constipation: still a long way to go. Dis Colon Rectum. 2009; 52:752–753. PMID: 19404088.
68. Kamm MA, Dudding TC, Melenhorst J, et al. Sacral nerve stimulation for intractable constipation. Gut. 2010; 59:333–340. PMID: 20207638.
69. Maeda Y, Lundby L, Buntzen S, Laurberg S. Sacral nerve stimulation for constipation: suboptimal outcome and adverse events. Dis Colon Rectum. 2010; 53:995–999. PMID: 20551750.
70. Ratto C, Ganio E, Naldini G. GINS. Long-term results following sacral nerve stimulation for chronic constipation. Colorectal Dis. 2015; 17:320–328. PMID: 25476039.
71. Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999; 230:627–638. PMID: 10561086.
72. Hassan I, Pemberton JH, Young-Fadok TM, et al. Ileorectal anastomosis for slow transit constipation: long-term functional and quality of life results. J Gastrointest Surg. 2006; 10:1330–1336. PMID: 17175451.
73. Glia A, Akerlund JE, Lindberg G. Outcome of colectomy for slow-transit constipation in relation to presence of small-bowel dysmotility. Dis Colon Rectum. 2004; 47:96–102. PMID: 14719156.
Table 1
New Pharmacological Treatments for Constipation
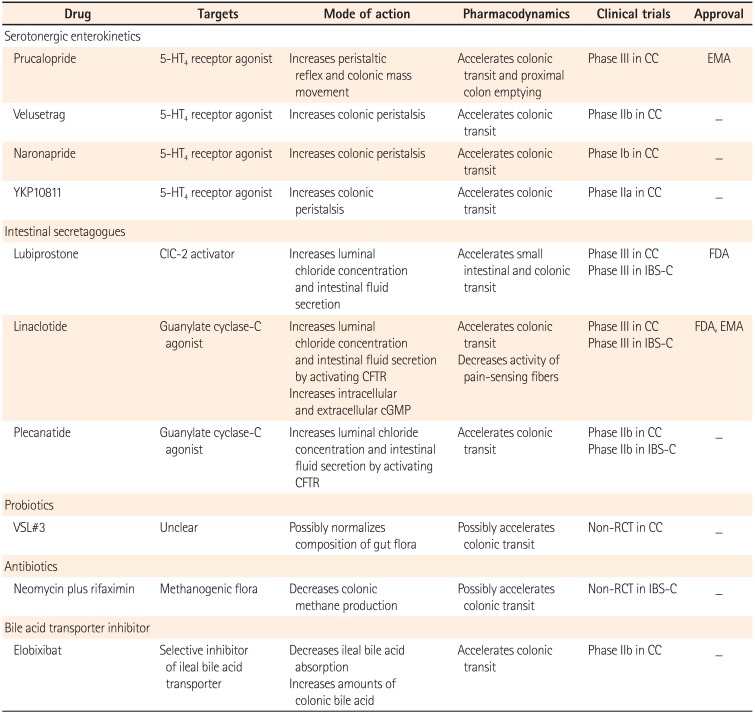
5-HT4, 5-hydroxytryptamine type 4; CC, chronic constipation; EMA, European Medicines Agency; ClC-2, type-2 chloride channel; IBS-C, IBS with constipation; FDA, Food and Drug Administration; CFTR, cystic fibrosis transmembrane conductance regulator; cGMP, cyclic guanosine-3',5'-monophosphate; RCT, randomized controlled trial.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download