Abstract
Familial Mediterranean fever (FMF) is an inherited autosomal recessive disorder, ethnically restricted and commonly found among populations surrounding the Mediterranean Sea. FMF is the most prevalent autoinflammatory disease; is characterized by recurrent, self-limited episodes of fever with serositis; and is caused by Mediterranean fever gene (MEFV) mutations on chromosome 16. We describe a case of adult-onset FMF with complete symptomatic remission during pregnancy, without the use of colchicine. A 25-year-old woman had presented with periodic fever, abdominal pain, and vomiting since she was 21. Her abdominal computed tomography scan showed intestinal nonrotation. She underwent exploratory laparotomy and appendectomy for her symptoms 1 year prior. She had a symptom-free pregnancy period, but abdominal pain and fever recurred after delivery. Mutation analysis of the MEFV gene revealed two point mutations (p.Leu110Pro and p.Glu148Gln). We report an adult female patient with FMF in Korea with complete symptomatic remission during pregnancy.
Familial Mediterranean fever (FMF) is an autosomal recessive disease characterized by symptoms such as recurrent episodes of fever, with serosal, synovial, or cutaneous inflammation.1 FMF is almost exclusively found in high-risk population groups, including those of Turkish, Armenian, Arabian, and Jewish descent in Mediterranean and Middle Eastern countries. The mutation of the Mediterranean fever gene (MEFV) located on the short arm of chromosome 16 plays a major role in the pathogenesis of this disease.2 The protein encoded by the MEFV gene is called marenostrin or pyrin, consisting of 781 amino acids, and is associated with the regulation of inflammation. In general, the age of onset is 20 years or younger in 90% of patients, 50% of whom have symptoms before age 10 years.3
The course of pregnancy in patients with FMF is variable. Some experience complete symptomatic remission, while others have more frequent attacks.4 Management of FMF attacks during pregnancy is strongly recommended, and continuous treatment with colchicine is recommended during pregnancy, even in patients with symptomatic remission.5
We report a case of adult-onset FMF with symptomatic remission during pregnancy in South Korea, who presented with recurrent periodic fever, abdominal pain, and vomiting.
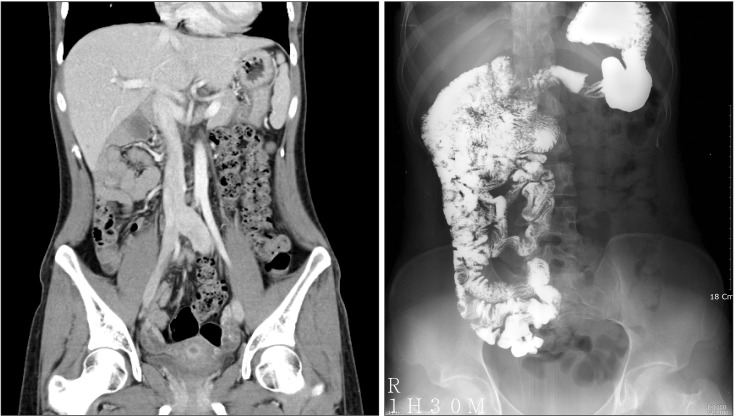
A 25-year-old woman visited our hospital because of fever, abdominal pain, and vomiting in December 2013. She had no histories of tobacco, alcohol, or illegal drug use, and travel outside Korea. She had had recurrent periodic fever, abdominal pain, and vomiting since she was 21 years. Symptoms recurred every 6 months at first, but the interval between symptoms gradually decreased to 3 months. She experienced intermittent attacks of abdominal pain, vomiting, nausea, and fever (39-40℃) for 3 to 5 days, and then the symptoms resolved spontaneously and completely. She had a normal daily life during the symptom-free period. A year prior to her visit in our hospital, she underwent several blood tests, abdominal CT, and gastroscopy in another hospital; her CT scan and small bowel follow-through (Fig. 1) showed complete intestinal nonrotation. Antibody screening test results for infection and autoimmune diseases were all negative. She underwent exploratory laparotomy and appendectomy in May 2012 to exclude adhesions due to intestinal nonrotation and other surgical causes of abdominal pain and fever. No significant adhesions or bowel torsion was observed. After surgery, she became pregnant, had no symptoms during pregnancy, and gave birth in March 2013. In December 2013, she again had abdominal pain and vomiting with fever and visited our hospital immediately after the first symptomatic attack since pregnancy.
Vital signs were blood pressure 110/73 mm Hg, pulse 80/min, respiratory rate 18/min, and body temperature 37.0℃ on admission. Mild diffuse abdominal tenderness was noted. Initial laboratory results showed leukocytes 9,200/mm3 (neutrophils 75.9%), hemoglobin 12.3 g/dL, platelets 218,000/mm3, and CRP 4.4 mg/L.
On the basis of her typical symptoms, FMF or tumor necrosis factor receptor-associated periodic syndrome were most likely among the periodic fever syndromes. We performed MEFV and TNFR1 genetic testing for the definitive diagnosis of FMF. Two missense mutations were detected in the exon 2 of the MEFV gene, a heterozygous mutation of c.329T>C (p.Leu110Pro) and a homozygous mutation of c.442G>C (p.Glu148Gln). Based on the presence of periodic fever and abdominal pain and the result of the MEFV gene mutation analysis, this patient was diagnosed with FMF.
The patient remains asymptomatic on colchicine 1.2 mg/day, but needs to be followed to confirm that colchicine controls recurrent symptoms.
FMF is an inherited, multisystem autosomal recessive disease, in which the mutation of the MEFV gene changes the function of a protein regulating the inflammatory response by blocking an intracellular pathway via nuclear factor κB or caspase-1.6 It is prevalent among Mediterranean ethnic groups, including those of Armenian, Arabian, Jewish, Turkish, and North African descent.3 The worldwide prevalence is estimated at 1/100,000-150,000. The prevalence of FMF is estimated to be 1/250-1,000 in non-Ashkenazi Jews, 1/73,000 in Ashkenazi Jews, and 1/500 Armenians.78 However, since the development of genetic testing, several case series of patients have recently been reported in unexpected and non-Mediterranean areas, including Japan9 and Korea.101112 Four cases were reported in Korea.10111213
A major symptom of FMF is recurrent periodic fever accompanied by pain in the abdomen, chest, or joints.6 Diagnosis is based on typical clinical features, response to colchicine, and genetic mutation analysis.14 The dominant manifestation of FMF is abdominal pain, with more than 90% of patients affected.3 The pain can range from mild to severe and may be diffuse or localized. During attacks, most patients may have initially localized pain that rapidly evolves into the clinical features of an acute abdomen more than functional abdominal pain. Abdominal symptoms are sometimes confused with peritonitis. Some patients with FMF undergo unnecessary abdominal surgery when they present with rebound tenderness, adynamic ileus, rigid abdomen, and fever. This patient with congenital intestinal nonrotation had complained of recurrent abdominal pain with vomiting and fever, which eventually led to unnecessary exploratory laparotomy to rule out adhesive ileus. However, congenital intestinal nonrotation was not a likely cause of this patient's abdominal symptoms.
FMF is a systemic illness affecting patients in their child-bearing years, and there is increased concern about its impact on the reproductive system.15 Some female FMF patients had menstruation-associated attacks that may be confused with gynecologic disorders.15 There are several studies of pregnancy outcomes in FMF.4516 The course of pregnancy in patients with FMF is variable. Among 73 pregnancies in 40 patients, 62.5% experienced complete symptomatic remission, 17.5% had more severe attacks, 15% had less severe attacks, and 5% had no change.4 This patient had complete symptomatic remission during pregnancy and lactation. The underlying pathophysiology of the relationship between FMF and menstruation or pregnancy is unknown. One possible explanation is that estrogen may play a role.15 There are several studies on the role of estrogen in inflammatory processes. An endogenous metabolite of 17β-estradiol that interacts with estrogen receptors and microtubules at or near the colchicine site suppresses lipopolysaccharide-induced interleukin-6 gene expression and PGE2 production,17 and interferes with normal microtubule function by suppressing microtubule dynamics.18 17β-estradiol can interfere with CRP proinflammatory molecules such as interleukin-8 and intercellular adhesion molecules.19 These effects are similar to those of colchicine, which also suppresses the expression of intercellular adhesion molecules and disrupts microtubule function, preventing frequent attacks and amyloidosis in FMF patients. Although some patients may experience complete symptomatic remission, control of FMF attacks with colchicine during pregnancy is strongly recommended, because peritonitis can lead to early contractions or abortion, and there is a risk of exacerbation of febrile attacks and development of amyloidosis.20
In conclusion, we report a female FMF patient who had experienced complete symptomatic remission during her pregnancy, even without colchicine, and who also had unnecessary exploratory laparotomy. The course of pregnancy in this patient suggests that hormonal changes may effect symptomatic remission. The relationship between FMF and pregnancy or menstruation is complex and needs further investigations. Physicians should consider a diagnosis of FMF as a rare cause of recurrent abdominal pain and vomiting accompanied by fever, even in non-endemic areas such as South Korea.
References
1. Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005; 17:586–599. PMID: 16093838.

2. The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997; 90:797–807. PMID: 9288758.
3. Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med. 1967; 43:227–253. PMID: 5340644.
4. Akar S, Soyturk M, Onen F, Tunca M. The relations between attacks and menstrual periods and pregnancies of familial Mediterranean fever patients. Rheumatol Int. 2006; 26:676–679. PMID: 16184383.

5. Yasar O, Iskender C, Kaymak O, Taflan Yaman S, Uygur D, Danisman N. Retrospective evaluation of pregnancy outcomes in women with familial Mediterranean fever. J Matern Fetal Neonatal Med. 2014; 27:733–736. PMID: 23981183.

6. Berkun Y, Ben-Chetrit E. Pyrin and cryopyrin - similar domain sequence but opposite inflammatory consequence. Clin Exp Rheumatol. 2007; 25(Suppl 45):S6–S8. PMID: 17949543.
7. Cattan D, Notarnicola C, Molinari N, Touitou I. Inflammatory bowel disease in non-Ashkenazi Jews with familial Mediterranean fever. Lancet. 2000; 355:378–379. PMID: 10665562.

8. Gershoni-Baruch R, Brik R, Shinawi M, Livneh A. The differential contribution of MEFV mutant alleles to the clinical profile of familial Mediterranean fever. Eur J Hum Genet. 2002; 10:145–149. PMID: 11938447.

9. Tomiyama N, Higashiuesato Y, Oda T, et al. MEFV mutation analysis of familial Mediterranean fever in Japan. Clin Exp Rheumatol. 2008; 26:13–17. PMID: 18328141.
10. Lim AL, Jang HJ, Han JW, et al. Familial Mediterranean fever: the first adult case in Korea. J Korean Med Sci. 2012; 27:1424–1427. PMID: 23166428.

11. Joo K, Park W, Chung MH, et al. Extensive thrombosis in a patient with familial Mediterranean fever, despite hyperimmunoglobulin D state in serum - first adult case in Korea. J Korean Med Sci. 2013; 28:328–330. PMID: 23400211.

12. Koo KY, Park SJ, Wang JY, et al. The first case of familial Mediterranean fever associated with renal amyloidosis in Korea. Yonsei Med J. 2012; 53:454–458. PMID: 22318840.
13. Lee CG, Lim YJ, Kang HW, et al. A case of recurrent abdominal pain with fever and urticarial eruption. Korean J Gastroenterol. 2014; 64:40–44. PMID: 25073670.

14. Soriano A, Manna R. Familial Mediterranean fever: new phenotypes. Autoimmun Rev. 2012; 12:31–37. PMID: 22878273.

15. Ben-Chetrit E, Ben-Chetrit A. Familial Mediterranean fever and menstruation. BJOG. 2001; 108:403–407. PMID: 11305548.

16. Turgal M, Selcuk I, Ozyuncu O. Pregnancy outcome of five patients with renal amyloidosis regarding familial Mediterranean fever. Ren Fail. 2014; 36:306–308. PMID: 24168456.

17. Shand FH, Langenbach SY, Keenan CR, et al. In vitro and in vivo evidence for anti-inflammatory properties of 2-methoxyestradiol. J Pharmacol Exp Ther. 2011; 336:962–972. PMID: 21177477.

18. Kamath K, Okouneva T, Larson G, Panda D, Wilson L, Jordan MA. 2-Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerizing microtubules. Mol Cancer Ther. 2006; 5:2225–2233. PMID: 16985056.

19. Cossette É, Cloutier I, Tardif K, DonPierre G, Tanguay JF. Estradiol inhibits vascular endothelial cells pro-inflammatory activation induced by C-reactive protein. Mol Cell Biochem. 2013; 373:137–147. PMID: 23111890.

20. Ben-Chetrit E, Levy M. Reproductive system in familial Mediterranean fever: an overview. Ann Rheum Dis. 2003; 62:916–919. PMID: 12972465.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download