INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract and typically arise from the interstitial cells of Cajal. Primary GISTs arise most commonly in the stomach (50-70%) or small bowel (20-30%), but may arise anywhere in the GI tract or rarely outside the GI tract as primary tumors.
1 The positivity for the c-kit (CD117) receptor tyrosine kinase, observed in approximately 95% of GISTs, is the molecular hallmark of GISTs. Widespread use of immunohistochemical staining for c-kit has now enabled accurate diagnosis.
2
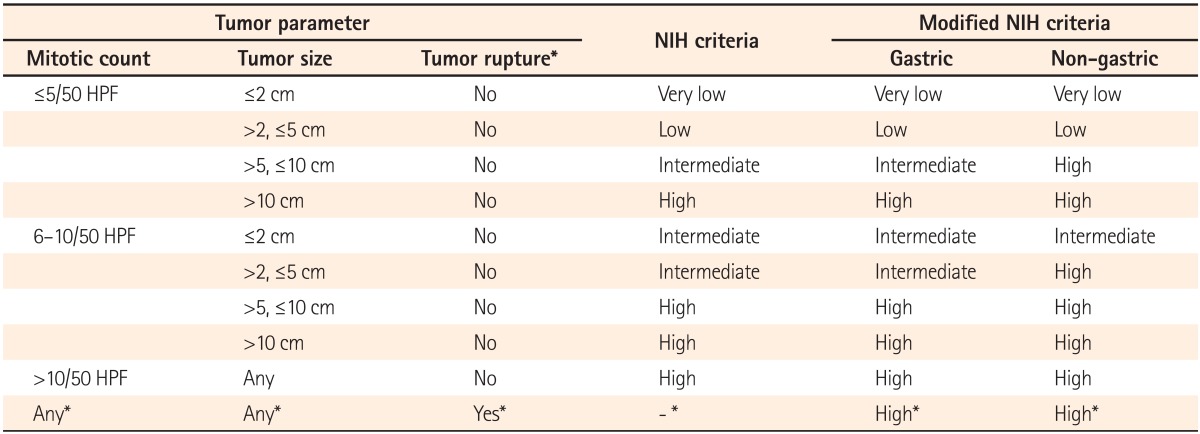
Surgical resection has been the gold standard for cure in the setting of localized GISTs. Although tumor recurrence is common and usually occurs in the liver and peritoneum, accurate assessment of the recurrence risk after curative resection is difficult. Many studies have focused on stratifying GISTs into prognostic categories based on different parameters; it is generally agreed that tumor size and mitotic count are important prognostic factors at all sites. Therefore, the National Institutes of Health (NIH) consensus criteria, which are based on tumor size and mitotic index, have been used to assess recurrence risk and to predict prognosis.
3 In a large study with long-term follow-up, the location of the primary tumor was an important prognostic factor. In that study, GISTs of the stomach showed a much lower rate of aggressive behavior than GISTs of the small bowel that were similar in size and/or mitotic rate.
4,
5,
6 Furthermore, tumor rupture became known as an independent risk factor that negatively impacted recurrence-free survival (RFS).
7,
8 Accordingly, a modification of the NIH criteria was proposed to distinguish prognosis after GIST resection (
Table 1).
3,
9
Table 1
Risk Stratification of Primary Gastrointestinal Stromal Tumors (GISTs) under the National Institutes of Health (NIH) Criteria and Modified NIH Criteria

The success of imatinib (Glivec®, Novartis Korea, Seoul, Korea) in the setting of advanced GIST prompted interest in its use in the adjuvant setting after complete resection of a primary tumor.
10,
11,
12 Recent studies showed that adjuvant imatinib therapy reduced the recurrence risk after curative resection of localized GISTs.
13,
14 However, improvement of RFS was marginal in patients with tumors <10 cm, although the recurrence risk was almost halved in patients with a primary tumor ≥10 cm.
15 Therefore, the need for accurate risk stratification has become increasingly important, because it is crucial for selecting patients who are most likely to benefit from adjuvant imatinib therapy.
16 Recent studies showed that the modified NIH criteria were the best classification to identify a single high-risk group for consideration of adjuvant therapy.
17,
18,
19 However, most of these studies were conducted in Western nations, and studies about the risk classification systems to predict prognosis after surgical resection in East Asian patients with GIST are scarce. Therefore, we performed this study to clarify the clinical usefulness of the modified NIH criteria, as compared with the original NIH criteria, in predicting recurrence of GISTs in the small intestine and colorectum after curative resection in Korea.
Go to :

METHODS
1. Patients
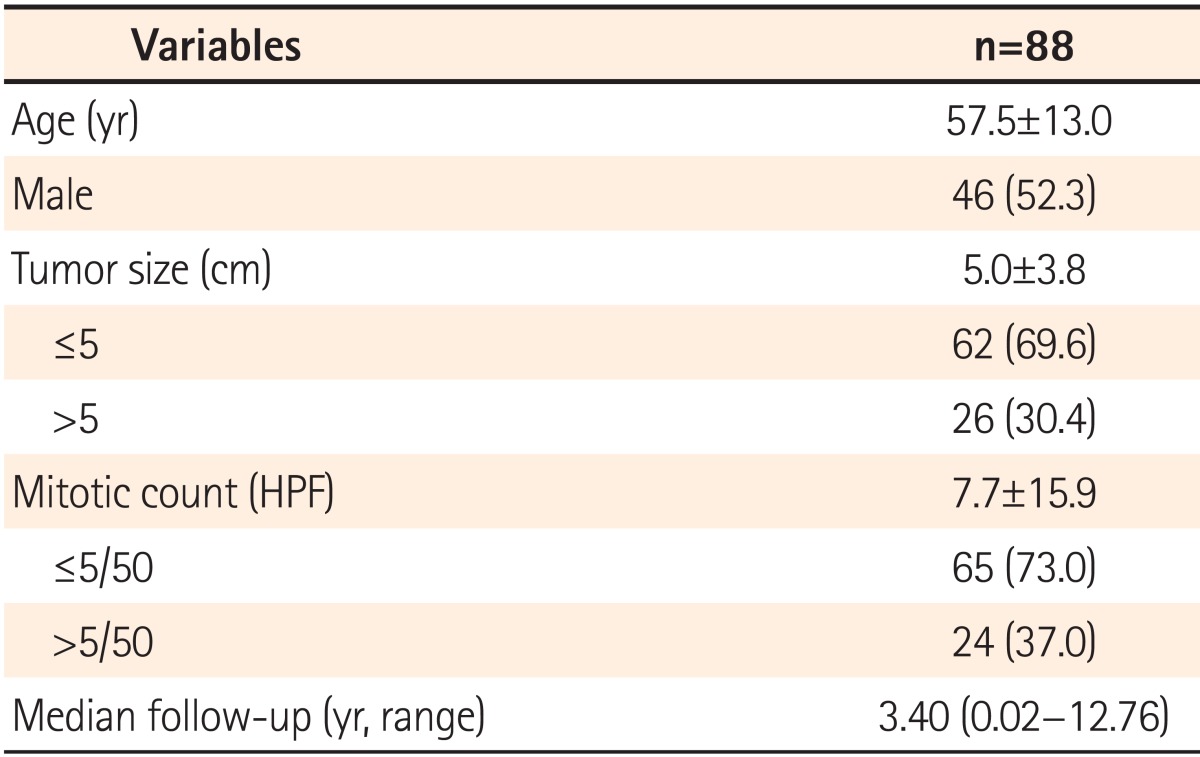
The patients who underwent curative surgery for primary GISTs of the small intestine and colorectum without metastasis between January 2000 and October 2012 at Seoul National University Hospital were enrolled. Those who received adjuvant chemotherapy including imatinib after surgery were excluded to observe the natural course of the tumor. Their clinical data regarding age, sex, tumor site, dates of surgery and the last follow-up, and pathologic reports including tumor size and mitotic rates were collected and analyzed retrospectively. The histological diagnosis of the surgical specimen was based on H&E and immunohistochemical staining for c-kit (CD117), CD34, S-100 protein, and smooth muscle actin. Patients were diagnosed with GISTs and included in this study if they had c-kit (CD117) and CD34-positive tumors with typical histologic patterns of spindle or epithelioid cell types. This study was approved by the institutional review board.
2. Risk Classification and Follow-up
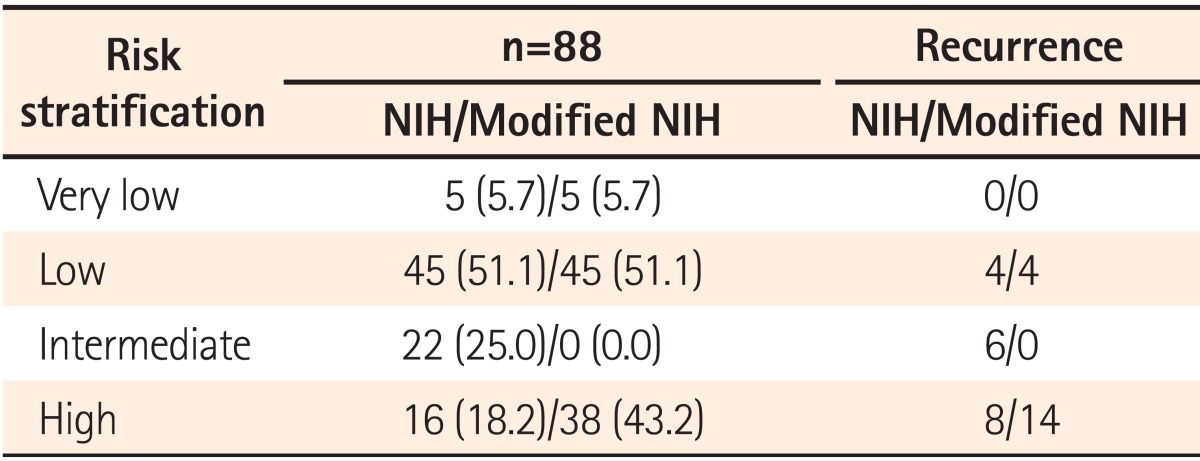
The original NIH criteria stratified tumors into the very low-, low-, intermediate-, and high-risk categories, based on tumor size and mitotic count. After modification of the NIH criteria, the site of the primary tumor and tumor rupture were included as prognostic factors (
Table 1). The patients were stratified into one of the four categories according to the NIH criteria and modified NIH criteria, and the changes of each risk category were analyzed.
Patients were followed to detect recurrence with endoscopy and/or an imaging modality including abdominal/pelvic CT on a regular basis of 6 to 12 months. RFS was defined from the date of radical surgery to the date of tumor recurrence.
3. Prognostic Factors
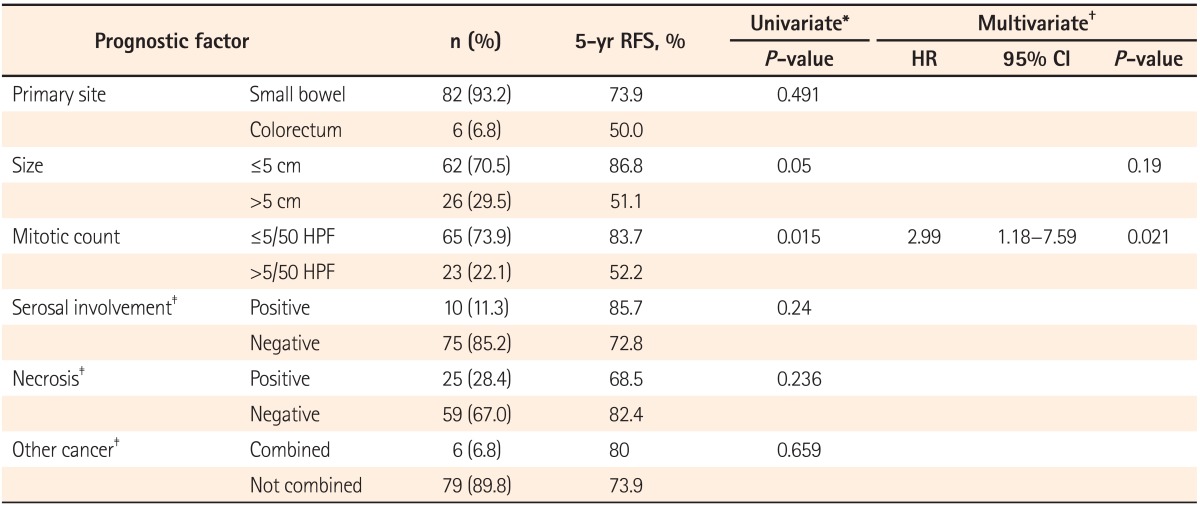
Tumor size and mitotic count are important prognostic factors at all sites. The site of origin, tumor rupture, and several histologic features, including serosal involvement and necrosis, are also known as prognostic factors. These factors were evaluated as potentially significant prognostic factors for tumor recurrence.
4. Statistical Analysis
Continuous variables were expressed as means±SD or median with range. Intergroup comparison of clinicopathological features was done with the Student's t-test. Categorical variables were compared with the Pearson's chi-square test. RFS was calculated with the Kaplan-Meier method, and the difference among groups was determined with the log-rank test. For the prognostic factors evaluation, each variable was assessed by performing univariate analysis. The variables with statistical significance were included in a multivariate analysis using the Cox regression hazard model. Results were considered statistically significant for P-values <0.05. Statistical analysis was performed with SPSS for Windows (version 18.0; SPSS Inc., Chicago, IL, USA).
Go to :

DISCUSSION
The present study confirmed that the high-risk group according to the modified NIH criteria significantly experienced more recurrence than the other groups of patients with GISTs in the small intestine and colorectum in Korea. Complete surgical resection remains the mainstay of treatment for patients with localized GISTs, but many patients have a substantial risk of recurrence in the liver, peritoneum, or both. Clinical outcomes for patients with recurrence were dismal, and conventional chemotherapy and radiation therapy achieved a significant response only in <10% of patients.
Imatinib, a tyrosine-kinase inhibitor, prolonged RFS after surgery for GIST in several studies and was approved as an adjuvant therapy for operable GISTs.
14,
20 In the National Comprehensive Cancer Network (NCCN) guidelines published in 2010, adjuvant imatinib therapy was highly recommended for patients with intermediate- to high-risk GISTs.
10 In addition, adjuvant imatinib was proposed as an option for those patients with a substantial risk of relapse in the European Society for Medical Oncology (ESMO) guidelines.
21 The Scandinavian Sarcoma Group (SSG) XVIII trial established that at least 36 months of adjuvant imatinib treatment had improved RFS and overall survival for patients with high-risk GISTs.
22 Thus, 3 years of imatinib treatment became a standard adjuvant therapy for patients with high-risk GISTs in the NCCN guidelines that were updated in 2012.
12
Until the ESMO guideline was published, "intermediate-to-high risk" and "substantial risk" were not clearly defined. In the SSG XVIII trial, "high risk" was defined as tumor diameter >5.0 cm and mitotic count >5/50 HPF; tumor diameter >10.0 cm; mitotic count >10/50 HPF; or tumors ruptured into the peritoneal cavity.
22 This definition of "high risk" is quite similar to that of the modified NIH criteria. Several studies were conducted to validate the risk stratification schemes, and the modified NIH criteria were identified as the most reliable tool for assessing prognosis of GIST as a result, especially for screening a single high-risk group.
17,
18
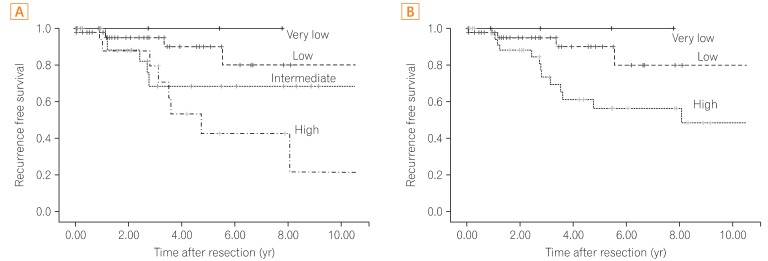
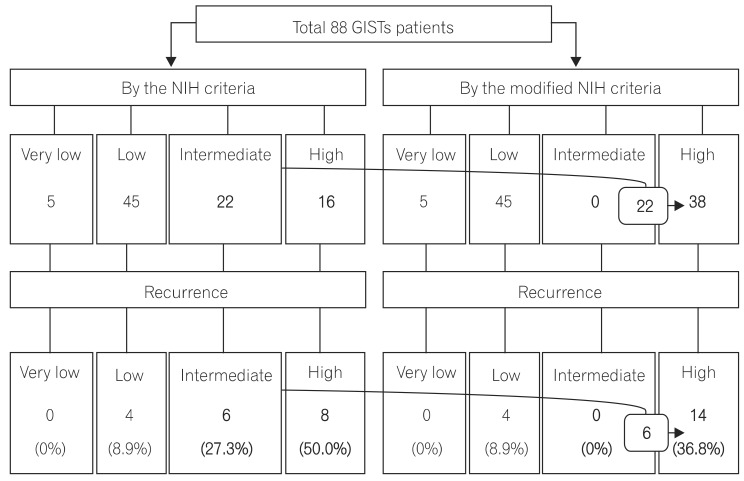
The present study corroborated a prognostic significance of the modified NIH criteria, which can be used effectively to determine whether individual patients need adjuvant imatinib therapy. As expected, both the very low- and low-risk categories had a favorable prognosis under the original NIH or modified NIH risk classification. Only 4 patients experienced recurrence among 50 patients in the very low- and low-risk groups. According to the modified NIH criteria, all 22 patients with intermediate risk under the original NIH criteria were reclassified to high risk. All 6 patients who experienced tumor recurrence were reclassified as high-risk patients. Thus, postoperative surveillance for recurrence without adjuvant therapy can be recommended to the very low-, low-, and intermediate-risk patients according to the modified NIH criteria, considering the potential toxicities and financial cost of the imatinib treatment. However, recurrence also can occur in the very low-, low-, and intermediate-risk patients, so regular follow-up with radiographic examination for recurrence surveillance is strongly recommended.
21
The 5-year RFS rates of very low/low-, intermediate-, and high-risk patients were inferior to those of a published meta-analysis, which were 91.0%, 69.0%, and 43.0%, respectively.
21 This result may be related to the poor prognosis of GISTs in the small intestine and colorectum.
Recurrence in the high-risk group was uniformly distributed during the follow-up period, and this study proved the necessity of adjuvant therapy in that group. According to the Kaplan-Meier curves of the 5-year RFS, it may appear that the original NIH criteria are a better stratification system than the modified NIH criteria. Actually, the recurrence rates were statistically significantly different between the very low/low- (8.0%, 4/50), intermediate- (27.3%, 6/22), and high-risk (50.0%, 8/16) categories according to the original NIH criteria during the follow-up period (median follow-up: 3.40 years, P=0.036). However, the most important aspect of the risk stratification system is to provide clinically useful standards. The main concern for patients with resectable GISTs is how to determine the patients who will need adjuvant chemotherapy after surgery. As previously described, the modified NIH criteria can determine the single most high-risk patient group for recurrence.
This study has several limitations including its single-center, retrospective design. In addition, recurrence developed only in 18/88 patients (20.5%), which was less than in previous studies and might be related to the small proportion of the high-risk group. In multivariate analysis, the mitotic count was a significant prognostic factor, but the primary tumor size was not, which might be related to the small proportion of patients with recurrence. Although the modified NIH criteria can distinguish the single high-risk patient group for consideration of adjuvant imatinib therapy, it cannot discriminate risk of recurrence between the very low-, low-, and intermediate-risk groups and cannot provide a quantifiable risk of recurrence for individual patients.
Accordingly, the modified NIH criteria predicted the recurrence of GIST after curative resection well, especially in the high-risk patients. Large population-based studies are needed to prove the relationship between recurrence and known prognostic factors in patients with GISTs in the small intestine and colorectum. Further studies of additional variables to predict the recurrence more precisely in order to individualize management are warranted.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download