초록
Background
Clostridial bacteremia (CB) is the second most frequent anaerobic bacteremia, and CB patients show high mortality without prompt antimicrobial therapy. We retrospectively reviewed 11 years of CB cases in a tertiary care hospital to describe the clinical and microbiological characteristics of CB and to de fi ne the risk factors of fatal CB.
Methods
All patients with CB from January 2002 to December 2012 were included in the study. Age, sex, underlying diseases, antibiotic use, and clinical outcome were reviewed. Antibiotic therapy was clas-sified as either ‘appropriate’ or ‘inappropriate’ based on the activity against Clostridium species.
Results
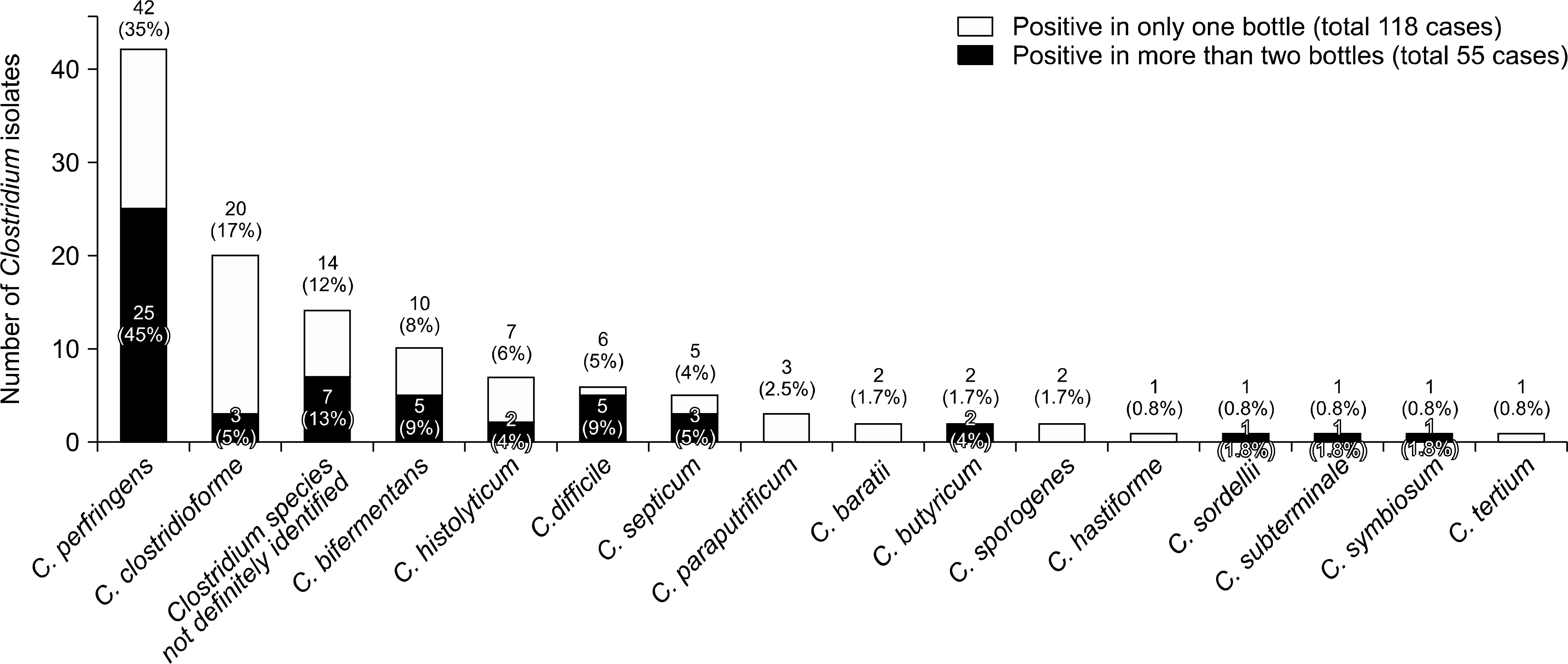
A total of 118 Clostridium isolates (0.79% of all blood culture isolates) were recovered from the blood cultures of 114 patients. The underlying con-ditions of patients with CB were neoplasm in 87 cases (76.3%), gastrointestinal symptoms in 84 cases (73.7%), diabetes in 17 cases (14.9%), and hemodialysis in six cases (5.3%). Of the 118 Clostridium isolates, C. perfringens was the most frequent species (42 isolates,35.6%). Thirty-two patients (28.1%) showed polymicrobial bacteremia, which was most commonly combined with Escherichia coli. Two patients harbored more than two Clostridium species. ‘Appropriate’ antibiotics were given to 97 (85.1%) patients. The mortality rate of CB at days 2, 8, and 30 was 7.9% (9/114), 14.0% (16/114), and 26.3% (30/114), respectively.
REFERENCES
2.Lassmann B., Gustafson DR., Wood CM., Rosenblatt JE. Reemer-gence of anaerobic bacteremia. Clin Infect Dis. 2007. 44:895–900.

3.Brook I. Anaerobic bacterial bacteremia: 12-year experience in two military hospitals. J Infect Dis. 1989. 160:1071–5.

5.Chen YM., Lee HC., Chang CM., Chuang YC., Ko WC. Clostridium bacteremia: emphasis on the poor prognosis in cirrhotic patients. J Microbiol Immunol Infect. 2001. 34:113–8.
6.de Virgilio C., Klein S., Chang L., Klassen M., Bongard F. Clostridial bacteremia: implications for the surgeon. Am Surg. 1991. 57:388–93.
7.Bodey GP., Rodriguez S., Fainstein V., Elting LS. Clostridial bacteremia in cancer patients. A 12-year experience. Cancer. 1991. 67:1928–42.

8.Gorbach SL., Thadepalli H. Isolation of Clostridium in human infections: evaluation of 114 cases. J Infect Dis. 1975. 131(Suppl):S81-5.
9.Benjamin B., Kan M., Schwartz D., Siegman-Igra Y. The possible significance of Clostridium spp. in blood cultures. Clin Microbiol Infect. 2006. 12:1006–12.
10.Rechner PM., Agger WA., Mruz K., Cogbill TH. Clinical features of clostridial bacteremia: a review from a rural area. Clin Infect Dis. 2001. 33:349–53.

11.Johnson S., Driks MR., Tweten RK., Ballard J., Stevens DL., Ander-son DJ, et al. Clinical courses of seven survivors of Clostridium septicum infection and their immunologic responses to alpha toxin. Clin Infect Dis. 1994. 19:761–4.
12.Shah M., Bishburg E., Baran DA., Chan T. Epidemiology and outcomes of clostridial bacteremia at a tertiary-care institution. Scien-tificWorldJournal. 2009. 9:144–8.

13.Stevens DL., Bryant AE., Carroll KC. Clostridium. Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, editors. Manual of Clinical Microbiology. 11th ed. Washington, D.C.: ASM Press;2015. p. 940–66.
14.CLSI. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. CLSI document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute. 2014.
15.Haddy RI., Nadkarni DD., Mann BL., Little DR., Domers TD., Clover RD, et al. Clostridial bacteremia in the community hospital.
16.Leal J., Gregson DB., Ross T., Church DL., Laupland KB. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000-2006. J Infect. 2008. 57:198–203.
17.Zahar JR., Farhat H., Chachaty E., Meshaka P., Antoun S., Nitenberg G. Incidence and clinical significance of anaerobic bacteraemia in cancer patients: a 6-year retrospective study. Clin Microbiol Infect. 2005. 11:724–9.

Table 1.
The frequency of clinical condition and 2-, 8-, 30-day mortality in each Clostridium species
| Type of Clostridium species (Total number) | DM | GI Symptom | HD | Neoplasms | Polymicrobial bacteremia | Healthcare-associated | Community-acquired | Mortality (2 day) | Mortality (8 day) | Mortality (30 day) |
|---|---|---|---|---|---|---|---|---|---|---|
| Clostridium perfringens (42) | 3 (7.1%) | 25 (59.5%) | 1 (2.4%) | 31 (73.8%) | 16 (38.1%) | 18 (42.9%) | 24 (57.1%) | 7 (16.7%) | 9 (21.4%) | 15 (35.7%) |
| Clostridium clostridioforme (20) | 3 (15%) | 16 (80%) | 2 (10%) | 16 (80%) | 3 (15%) | 7 (35%) | 13 (65%) | 0 (0%) | 5 (25%) | 7 (35%) |
| Clostridium species∗ (14) | 4 (28.6%) | 10 (71.4%) | 0 (0%) | 12 (85.7%) | 4 (28.6%) | 6 (42.9%) | 8 (57.1%) | 0 (0%) | 0 (0%) | 2 (14.3%) |
| Clostridium bifermentans (10) | 1 (10%) | 7 (70%) | 0 (0%) | 9 (90%) | 3 (30%) | 7 (70%) | 3 (30%) | 1 (10%) | 1 (10%) | 2 (20%) |
| Clostridium histolyticum (7) | 0 (0%) | 7 (100%) | 0 (0%) | 6 (85.7%) | 1 (14.3%) | 4 (57.1%) | 3 (42.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Clostridium difficile (6) | 1 (16.7%) | 6 (100%) | 1 (16.7%) | 5 (83.3%) | 3 (50%) | 3 (50%) | 3 (50%) | 0 (0%) | 0 (0%) | 1 (16.7%) |
| Clostridium septicum (5) | 1 (20%) | 4 (80%) | 0 (0%) | 3 (60%) | 1 (20%) | 1 (20%) | 4 (80%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Clostridium paraputrificum (3) | 1 | 3 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 |
| Clostridium baratii (2) | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| Clostridium butyricum (2) | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Clostridium sporogenes (2) | 2 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Clostridium hastiforme (1) | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Clostridium sordellii (1) | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Clostridium subterminale (1) | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Clostridium symbiosum (1) | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Clostridium tertium (1) | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
Table 2.
The proportion of patients with neoplasms in CB and non- CB cases during the 11-years study period
| Patient No. | CB | non-CB | Total |
|---|---|---|---|
| With neoplasms | 87 (76.3%) | 5,237 (51.1%) | 5,324 (51.4%) |
| Without neoplasms | 27 (23.7%) | 5,005 (48.9%) | 5,032 (45.6%) |
| Total | 114 | 10,242 | 10,356 |
Table 3.
Comparison of epidemiologic and clinical characteristics of polymicrobial and monomicrobial infection in patients with CB




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download