Abstract
Verruciform xanthoma (VX) is a rare, benign lesion that presents in the oral cavity, skin, or genital organs as a verrucous, papillomatous, or flat papule with varying colors. VX has indistinct clinical features, making histopathological examination necessary for a definitive diagnosis. Histologically, VX is characterized by parakeratosis, rete ridges with uniform depth, and an accumulation of the foam cells, which are also known as the "xanthoma cells". These foam cells test positive for antibodies, such as CD-68 and vimentin; it is thought that VX foam cells are derived from the monocyte-macrophage lineage, and that VX's pathogenic mechanism is partly related to an immune mechanism. Nevertheless, the pathogenesis of VX remains unclear. VX can be treated by surgical excision; other medical, chemical, and radiological treatments are not required postoperatively. Recurrence and malignant transformation of VX are rare. Two patients, each with a mass of unknown origin on the palatal gingiva, were presented at our clinic. Excisional biopsies of the masses were performed for a histological diagnosis after clinical and radiological examinations. Histological examination confirmed a diagnosis of VX in both cases.
Verruciform xanthoma (VX) is a rare, benign lesion that presents in the oral cavity, skin, or genital organs. It grows slowly, usually without causing pain, and its appearance resembles local epithelial hyperplasia such as verruciform papules, papilla, and plaque1. The color of VX can vary between white, pink, and yellow, depending on the thickness of the overlying epidermis. VX contains foam cells, which are lipid-containing histiocytes found within the submucosal substrates2,3. These findings are similar to those observed in leukoplakia, papilloma, verruca vulgaris, condyloma acuminata, and certain malignant lesions, and it is thus necessary to differentiate VX from these lesions with a tissue biopsy4,5.
The development of VX is thought to be related to a non-specific reaction to local epithelial trauma or an immune reaction. However, the etiology and pathogenesis of VX are not yet fully understood6. VX can be treated with conservative resection. Recurrence and malignant transformation of VX are rare, and the disorder usually has a good prognosis3.
So far, only a few cases of VX presenting in the oral cavity have been reported4. We report two cases of VX that presented on the maxillary palatal gingiva of male patients and review the relevant literature on this disorder.
A 37-year-old man visited our hospital (Gangnam Severance Hospital in Seoul, Korea) because of a poorly healing wound on his palate. The patient accidentally discovered the wound one year prior to his first visit. Although the patient did not experience any pain, he described the lesion as being somewhat uncomfortable or sensitive when irritated by food or palpation. The patient was advised to undergo a biopsy even though the lesion did not seem to progress and change in size, shape, or position.
The patient did not report any significant past medical history, history of trauma, or exceptional medical or family history. He reported a 13-year smoking history but no other specific social habits. During the intraoral examination, a well-demarcated soft and pedunculated papule with a verruciform papillary surface was found on the left side of the hard palate. The lesion was approximately 1.5 cm in diameter, reddish in color, and clinically asymptomatic.(Fig. 1) No other intraoral or skin lesions were identified. During the radiological examination, complete with periapical and panoramic radiographs, no specific findings were observed.(Fig. 2) Considering the results of the clinical and radiographic examinations, we identified multiple provisional diagnoses of this lesion, including leukoplakia, papilloma, condyloma acuminatum, and malignancies such as squamous cell carcinoma or verrucous carcinoma. Given the lesion's size, history, well-demarcated margin, and the results of a neck node examination, however, the probability of malignancy was quite low. An excisional biopsy, rather than an incisional biopsy, was then planned and performed in order to resect the total lesion and obtain a definitive diagnosis. Histopathological examination revealed a papillary proliferation of the stratified squamous epithelial cell layer, which was covered with a hyperkeratotic layer of uniform thickness, about that of the stratum spinosum.(Fig. 3. A) Distinctive foam cells, also referred to as xanthoma cells, as well as macrophages with vacuolated cytoplasm could be seen in the connective tissue layer when magnified under a high-resolution microscope.(Fig. 3. B)
After the lesion was totally resected, the surgical site underwent normal secondary healing processes. The patient had no complications, and there was no evidence of recurrent disease during the three-year follow-up period.(Fig. 4)
A 35-year-old man was referred to our clinic for evaluation of a lesion that developed on his hard palate. The patient became aware of the lesion one month prior to his first visit. During consultation, he complained that the lesion became sensitive when it came in contact with hot and salty foods, but he experienced no other specific symptoms. The patient had a history of an unknown sexually transmitted disease, which he contracted one year prior to his first visit and completely recovered from by the time of consultation. In addition, the patient had a 20-year history of heavy smoking. No other specific general diseases or exceptional medical history were noted. During the clinical examination, which was conducted during his first consultation, a soft sessile mass with a verrucous, papillary surface resembling a strawberry was found on the palatal gingiva of the left maxillary second premolar and first molar. The lesion was 2.5×1.0 cm in size, well demarcated, and pinkish in color. It was not painful when palpated. He also had a normal response in the percussion, mobility, and vitality tests of his teeth, however; the palatal aspect of his left maxillary first molar showed mild gingival recession. The radiological examination, including periapical and panoramic radiographs, did not yield specific results. Further, there was no enlargement or induration of his bilateral neck lymph nodes on palpation. Though we could not obtain more information about his medical history in regard to his sexually transmitted disease, we suspected a relationship. We diagnosed the lesion with differentials that included syphilis and malignancies such as squamous cell carcinoma or verrucous carcinoma. An incisional biopsy for a definitive diagnosis was performed one week after the initial examination. During histopathologic examination, histology slides obtained from the biopsy showed a papillary squamous epithelial cell layer covered with parakeratotic surface (Fig. 5. A) and xanthoma cells in the subepithelial connective tissue layer (Fig. 5. B), distinctive features of VX. Conservative surgical resection was performed after the results of the biopsy were confirmed. Postoperative follow-up examination showed a well-healed surgical site, and the patient had no complaints.
Four years after this resection, the patient returned to our clinic for the recurrent appearance of the lesion at the same site. The lesion appeared to have grown to a similar size, and its shape was also similar to the one resected previously. Further, the recurring mass affected the same part of the buccal gingiva around his left maxillary second premolar and first molar. Once a clinical diagnosis of a recurrent lesion of the pre-existing VX was made, a wide surgical resection, which included seemingly normal tissues surrounding the lesion, was performed. The results of the histopathologic examination of the recurrent lesion were the same as that of the initial lesion. The patient has been followed-up at regular intervals of fewer than six months for the past ten years, and there has been no evidence of VX recurrence.
VX is a relatively rare benign lesion that was first studied and defined by Shafer1 in 1971. Since then, the presence of VX in the oral cavity has been reported in about 300 cases4, while only five cases of VX-related skin lesions have been reported in South Korea6,7. Although there have been no definite studies on the incidence of this disease, a prevalence of approximately 0.025% to 0.094% has been reported4,5. To date, only about ten cases of VX have been reported in Asian and African-American studies, with the majority of patients being Caucasian1-5. VX commonly occurs during middle age (between 40 and 60 years of age), but it can also develop in other age groups. Further, it has no specific sex predominance4,5. The oral cavity and the genital mucosa are the most affected regions, and VX usually appears as a single lesion. VX can arise in any part of the oral cavity, although the most commonly affected sites are, the gingiva, hard palate, tongue, and buccal mucosa, in order of commonality4. Although multiple VX lesions have been reported in several patient groups with systemic lipid metabolic abnormalities, there has, in general, been no definite correlation between VX and systemic diseases8. Furthermore, VX does not seem to have a specific relationship with environmental factors such as a history of smoking or drinking or the use of prosthetics such as dentures5.
The name of the disease implies its clinical characteristics as well as its histological diagnosis. VX that develops within the oral mucosa presents clinically as a single indolent lesion with a surface of flat papillary or verruciform protrusions. Such lesions are usually about two cm in diameter and can have various colors including gray, pink, and yellow depending on the thickness and properties of the overlying epidermis1-5. It is essential to obtain a tissue biopsy for a definitive diagnosis of VX because it is difficult to clinically differentiate between VX and other diseases involving verruciform surfaces. Other conditions with similar clinical features that should be considered are leukoplakia, papilloma, verruca vulgaris, condyloma acuminata, and malignant lesions such as squamous cell carcinoma or verrucous carcinoma with verruciform surfaces9,10.
Pathohistologically, VX presents with relatively specific characteristics. In general, hematoxylin-eosin staining shows a parakeratotic layer that extends to the papillary surface, while the epithelial rete peg has a generally even thickness with a prominent extension into the underlying connective tissue. A marked increase in epithelial cell division or pseudo-epithelial hyperplasia is not observed. Infiltration of chronic inflammatory cells that contain few neutrophils and cell groups consisting of several small and large cells with vacuolated cytoplasm are dominant in the dermal connective tissue layer between the extended rete pegs. These are specific foam cells called xanthoma cells, which could be the pathological and histological characteristics of a definitive diagnosis for VX2. When stained by scharlach red, xanthoma cells present with an orange-red color typical of lipids, and several small and large lipid granules can be seen in the cytoplasm when histology slides are viewed under a high-resolution microscope. Other features of VX such as swelling of the mitochondria, extension of the Golgi apparatus, expansion of the endoplasmic reticulum, and destruction of the junctional complexes can also be observed under a high-resolution microscope. Histological features of VX, including hyperkeratosis, infiltration of neutrophils, and foam cells, are regularly seen in other injured cells, and it is therefore thought that VX starts with epithelium atrophy and destruction and then proceeds to the generation of foam cells11. This premise is supported by reports that show that VX is associated with diseases such as lichen planus, lupus erythematosus, vesicular epidermolysis bullosa, pemphigus vulgaris, and graft-versus-host disease8,11,12.
In 2005, Hu et al.13 conducted immunohistochemical tests using various monoclonal antibodies in an effort to identify the pathogenesis of VX. The authors concluded that foam cells in VX tested positive for specific antibodies of CD68 and vimentin, and that macrophages and immune cells in the monocyte system fulfilled a role in the immune mechanism. However, Hu et al.13 could not identify a clear pathogenesis for VX. Thus far, various studies have presented possible factors that could cause VX, including microbial community, human papillomavirus, lymphedema, genetic tendency, and dermal trauma with an inflammatory response11,13,14. Nevertheless, to our knowledge, no published study has identified significant correlations between these factors and the occurrence of VX. Thus, it is necessary to investigate such correlations in the future.
VX can be treated with conservative resection, and it does not require medical, chemical, or radiological treatment after surgery. Recurrent VX and malignant transformations are rare. In this case presentation, we report one case of recurrence four years after conservative surgical resection. After the second surgical procedure, VX did not recur in a follow-up period longer than ten years. The reported cases also show that the prognosis of VX is relatively good1-5,9,10,15.
As VX is a rare benign tumor, general practitioners and even specialists such as oral and maxillofacial surgeons are rarely aware of this disease. Thus far, there have been very few reports of VX. Considering that there are a limited number of studies investigating the relationship among an immunologically compromised status, systemic diseases, and VX, we deem it necessary to improve the knowledge of VX and have therefore reported two cases of VX treated at our clinic.
References
1. Shafer WG. Verruciform xanthoma. Oral Surg Oral Med Oral Pathol. 1971; 31:784–789. PMID: 5280461.

2. Zegarelli DJ, Zegarelli-Schmidt EC, Zegarelli EV. Verruciform xanthoma. Further light and electron microscopic studies, with the addition of a third case. Oral Surg Oral Med Oral Pathol. 1975; 40:246–256. PMID: 1057149.
3. van der Waal I, Kerstens HC, Hens CJ. Verruciform xanthoma of the oral mucosa. J Oral Maxillofac Surg. 1985; 43:623–626. PMID: 3859615.

4. Philipsen HP, Reichart PA, Takata T, Ogawa I. Verruciform xanthoma--biological profile of 282 oral lesions based on a literature survey with nine new cases from Japan. Oral Oncol. 2003; 39:325–336. PMID: 12676251.

5. Yu CH, Tsai TC, Wang JT, Liu BY, Wang YP, Sun A, et al. Oral verruciform xanthoma: a clinicopathologic study of 15 cases. J Formos Med Assoc. 2007; 106:141–147. PMID: 17339158.

6. Nam YH, Oh CW. A case of verruciform xanthoma presenting as leukoplakia on the lower lip. Korean J Dermatol. 2006; 44:508–511.
7. Choi YH, Moon YJ, Lee YW, Won JY, Song ES. A case of multiple verruciform xanthoma of oral cavity and gastrointestinal tract. Korean J Dermatol. 2002; 40:162–165.
8. Travis WD, Davis GE, Tsokos M, Lebovics R, Merrick HF, Miller SP, et al. Multifocal verruciform xanthoma of the upper aerodigestive tract in a child with a systemic lipid storage disease. Am J Surg Pathol. 1989; 13:309–316. PMID: 2539022.

9. Mete O, Kurklu E, Bilgic B, Beka H, Unur M. Flat-type verruciform xanthoma of the tongue and its differential diagnosis. Dermatol Online J. 2009; 15:5. PMID: 19930992.

10. Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & maxillofacial pathology. 2nd ed. Philadelphia: WB Saunders;2002.
11. Cumberland L, Dana A, Resh B, Fitzpatrick J, Goldenberg G. Verruciform xanthoma in the setting of cutaneous trauma and chronic inflammation: report of a patient and a brief review of the literature. J Cutan Pathol. 2010; 37:895–900. PMID: 19958440.

12. Shahrabi Farahani S, Treister NS, Khan Z, Woo SB. Oral verruciform xanthoma associated with chronic graft-versus-host disease: a report of five cases and a review of the literature. Head Neck Pathol. 2011; 5:193–198. PMID: 21305367.
13. Hu JA, Li Y, Li S. Verruciform xanthoma of the oral cavity: clinicopathological study relating to pathogenesis. Report of three cases. APMIS. 2005; 113:629–634. PMID: 16218939.

14. Mostafa KA, Takata T, Ogawa I, Ijuhin N, Nikai H. Verruciform xanthoma of the oral mucosa: a clinicopathological study with immunohistochemical findings relating to pathogenesis. Virchows Arch A Pathol Anat Histopathol. 1993; 423:243–248. PMID: 8236821.

15. Visintini E, Rizzardi C, Chiandussi S, Biasotto M, Melato M, Di Lenarda R. Verruciform xanthoma of the oral mucosa. Report of a case. Minerva Stomatol. 2006; 55:639–645. PMID: 17211369.
Fig. 1
Clinical aspect of the palate lesion in Case 1 on the first visit. A reddish papule with a rough verrucous surface and distinctive margin can be observed.
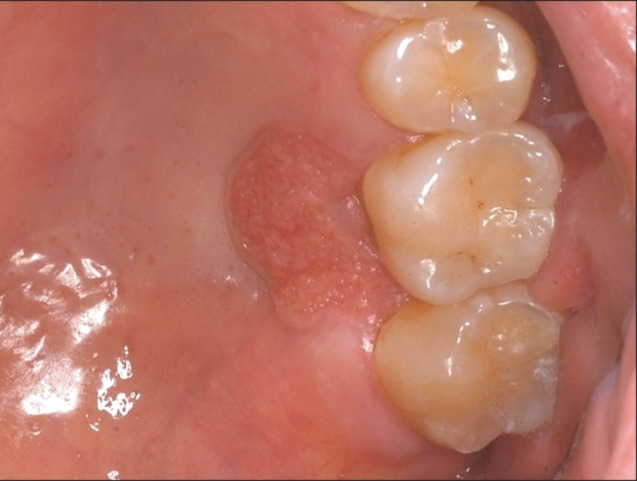
Fig. 2
Radiological examination of Case 1. A. Periapical view. B. A magnified view of orthopantogram. No specifing findings on these two radiograph images.
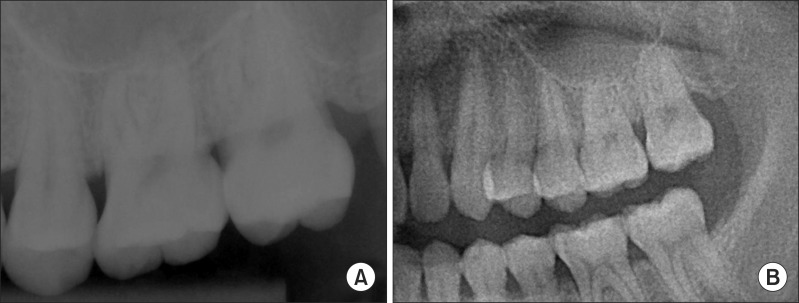
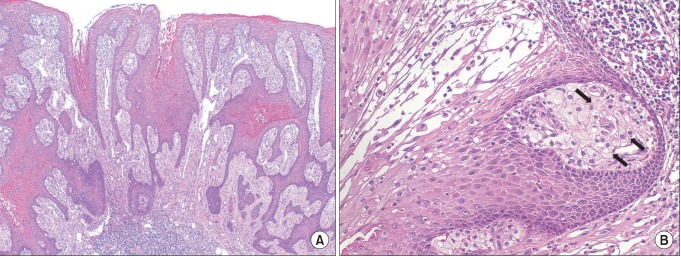
Fig. 3
Histopathological examination of Case 1 after excisional biopsy. A. The tissue has a papillary appearance with hyperparakeratosis and uniform rete peg (H&E staining, ×40). B. High magnification (H&E staining, ×200) illustrates large foam cells (xanthoma cells, black arrows) in the connective tissue papillae.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download