Abstract
Objectives
Angiogenesis and lymphangiogenesis are correlated with tumor growth and lymph node metastasis in cases of oral squamous cell carcinoma (OSCC). Endoglin is one of the representative vascular endothelial cell markers. Podoplanin is also a representative marker used in order to detect lymphatic endothelial cells. The aim of this study was to determine the correlation between the expression of endoglin/podoplanin and clinical variables associated with OSCC progression.
Materials and Methods
Paraffin embedded tissue specimens from 21 patients diagnosed with OSCC were used in this study. Ten patients were diagnosed with early clinical stage (I or II) and 11 patients with advanced clinical stage (III or IV) OSCC. Five patients had positive lymph node involvement. Primary antibodies for endoglin and podoplanin were used to perform the immunohistochemical detection of the vascular and lymphatic endothelial cells. The expression of endoglin and podoplanin was examined by an image analysis program in the three most highly expressed regions of each specimen.
Results
The average endoglin expression was observed to be 1.691±0.920 in the advanced stage (III, IV) specimens and 0.797±0.583 in the early stage (I, II) specimens (P=0.020). The average expression of podoplanin was 0.286±0.228 in the advance stage (III, IV) specimens and 0.374±0.157 in the early stage (I, II) specimens (P>0.05). There was no statistically significant difference in the expression of endoglin and podoplanin, regardless of whether or not the lymph node was positive.
Oral squamous cell carcinoma (OSCC) is the most common type of head and neck cancer1. It results from diverse genetic mutations of oral epithelial cells. The initiated and promoted cancer cells generally have a monoclonal feature. In the progression stage, however, it mostly has heterogeneity due to the further genetic modifications of the cancer cells2,3. Thus, each of OSCC appears to have biologically diverse patterns and clinically various behaviors4.
There are many kinds of treatment strategies depending on the predicted prognosis of OSCC5. The prediction of its prognosis can be helpful to clinicians in establishing a proper treatment plan. If some cancers are predicted to be aggressive behaviors, clinicians must consider a more aggressive treatment plan6. The TNM staging system has been widely used for the classification of OSCC according to its expected survival rate. This system is a simple method using the tumor size including resectability (T), lymph node involvement (N), and distant metastasis (M). It helps clinicians predict effectively the cancer prognosis and plan the treat ment strategy. This system is generally accepted as a good prognostic indicator for head and neck cancer7.
As a different staging system, specific molecular markers derived from cancer tissue have been applied in recent years for the diagnosis and prediction of OSCC prognosis. Many studies have been conducted to discover useful molecular markers for clinical applications6. Some molecular markers are practically used as tools for adjunctive purposes to evaluate the behavior of OSCC. For instance, epidermal growth factor receptor, cyclin D1, P53, and Matrix metalloproteinase are well-known molecular markers in OSCC. These are related to either cell proliferation or tumor suppression or matrix degradation4,8.
Micro-vessels are an essential part of the growth and progression of a tumor in terms of providing nutrients9,10. In a similar fashion, lymphatic vessels are also an important factor in the lymphatic metastasis of tumor cells11,12. In this study, endoglin and podoplanin as molecular markers were evaluated in tissue specimens from OSCC. Endoglin is one of the representative vascular endothelial cell markers; podoplanin is also a specific marker of lymphatic endothelial cells13,14. Micro-vessels and lymphatic-vessels can be identified by using the primary antibodies for endoglin and podoplanin, respectively. This study was performed to confirm that the formation of micro-vessels and lymphatic vessels is actually correlated with tumor growth and metastasis. If the expressions of endoglin and podoplanin are closely correlated with the clinical stage of OSCC, these markers could be used to evaluate the progression of OSCC. Therefore, this study sought to determine the correlation between the expression of endoglin/podoplanin and the clinical variables of OSCC.
Paraffin specimens from 21 patients diagnosed with OSCC were included in this study. Ten patients were in the early clinical stage (I or II), and eleven patients were in the advanced clinical stage (III or IV). Five patients had positive lymph node involvement, and sixteen patients had negative lymph node involvement by cancer.(Table 1) The exclusion criteria for this study were as follows: (1) recurrent cases of OSCC, and (2) patients with history of adjuvant or curative chemo/radiotherapy before the surgical resection of the main mass. Four surgeons were involved in the surgery of these patients. The patients' charts were reviewed to obtain information such as age, sex, treatment modalities, tumor location, tumor size, lymph node involvement, and distant metastasis.
Hematoxylin and eosin (H&E) staining and immunohistochemical staining were done for the histological examination. Paraffin-embedded tissue blocks were sliced to thickness of 4 µm. The sections of each tissue were carefully located on the silane-coated slides. These slides were incubated at 60℃ for 1 hour. After cooling to room temperature, the tissue slides were soaked in 100% xylene for 5 minutes in triplicate. At the final step of the xylene treatment, these slides were soaked for a longer time to remove the paraffin completely. The tissue sections were then hydrated by the consecutive application of high- to low-grade ethyl alcohol. Fully hydrated tissue sections were washed with distilled water, and then stored in phosphate-buffered saline solution (PBS, pH 7.5). PBS solutions were applied to wash and preserve the tissue sections in between steps. A universal LSAB+kit (Dako, Glotstrup, Denmark) was used for the immunohistochemical staining. For the antigen, the retrieval procedure was not done. Hydrated tissue sections were treated with 3% hydrogen peroxide for 15 minutes at room temperature to remove endogenous peroxidase and avidins. After that, serum-blocking solutions were applied for 20 minutes to prevent the binding of unspecific proteins. The following antibodies were used as primary antibodies against endoglin and podoplanin: (1) rabbit polyclonal to endoglin (prediluted) (ab27422; Abcam, Cambridge, MA, USA), and (2) mouse monoclonal to podoplanin (ab77854; Abcam). Endoglin was used with no dilution, and podoplanin was used with 1 : 40 dilution. Negative control specimens were not treated with primary antibodies. Tissue sections treated with primary antibodies were incubated overnight at 100% humidity in temperature chambers at 4℃. After adequate binding of the primary antibodies, biotinylated link solution as a secondary antibody was applied to the tissue sections for 30 minutes. After washing to remove any and all unbound secondary antibodies, streptavidin peroxidase solution was applied for 30 minutes. For colorization, diaminobenzidine solution was applied for less than 5 minutes. Mayer's hematoxylin was used for counter-staining. After gradational hydration with ethyl alcohol and clearing with xylene, the tissue sections were fixed by paramount solutions.
Tissue slides were observed with a microscope camera (Olympus DP70 digital microscope camera; Olympus Co., Tokyo, Japan). Positive control of endoglin and that of podoplanin were the human tonsil and the muscularis propria of human bowel, respectively. Images were taken from 3 most highly expressed regions (hot spot)15,16 per specimen under ×100 magnification. All images were blindly assessed to prevent bias of the region. To analyze the expression of endoglin and podoplanin, an image analysis program (SigmaScan Pro 5.0; SPSS Science, Chicago, IL, USA) was used17. The expression of endoglin or podoplanin was measured by the number of pixels, which appeared as red by this program. The average ratio of red pixels to the total pixels was used as variable for each specimen in the analysis.
Nonparametric tests were used to evaluate the expression of endoglin and podoplanin due to the small sample size. Mann-Whitney U tests were performed to analyze the correlation between the expression of endoglin or podoplanin and clinico-pathological parameters (age, sex, early or advanced stage of OSCC, and lymph node involvement). In addition, Spearman's rank correlation coefficient test was used to evaluate the relationship between the expression of endoglin and podoplanin in each of the OSCC tissue samples. Statistical analysis was performed with IBM SPSS statistics 19.0 (SPSS Inc., Chicago, IL, USA). The significant level of all statistics was set at P<0.05.
Endoglin and podoplanin, for the most part, were not co-localized in the same region of the serial sections.(Figs. 1. A, 1. B) The average expression of endoglin was 1.691±0.920 in the advanced stages (III, IV) and 0.797±0.583 in the early stages (I, II) (P=0.020). The average expression of podoplanin was 0.286±0.228 in the advanced stages (III, IV) and 0.374±0.157 in the early stages (I, II) (P=0.193). The average expression of endoglin was 1.677±1.246 in the positive lymph node involvement group and 1.137±0.748 in the negative lymph node involvement group (P=0.495). The average expression of podoplanin was 0.345±0.287 in the positive lymph node group and 0.319±0.173 in the negative lymph node group (P=1.000). There was no significant correlation between the expression of endoglin and podoplanin and age or gender. The average expression of endoglin and podoplanin of the entire specimen was 1.266±0.886 and 0.326±0.199, respectively. The correlation coefficient between the expression of endoglin and podoplanin was -0.304 (P=0.193).(Table 2)
Many studies have demonstrated that angiogenesis and lym phan giogenesis are correlated with tumor growth and lymphatic invasion in various malignancies including OSCC9-12. Thus, angiogenesis and lymphangiogensis in malignant tissues can be targeted for cancer treatment18-20. In the case of beva cizumab, the vascular endothelial growth factor inhibitor is clinically effective in inhibiting angiogenesis. It has been used as adjuvant to the main chemo-agent in the treatment of breast, lung, and colorectal cancers21-24.
In this study, the primary antibodies for endoglin and podoplanin were used to detect vascular and lymphatic endothelial cells, respectively, in OSCC. Endoglin is a glycoprotein mostly located in the membrane of vascular endothelial cells25. As a component of the transforming growth factor-beta (TGF-β) receptor, it is involved in the TGF-β signaling pathway26. The precise mechanism of its action is still unclear. Note, however, that endoglin is generally known to be linked closely to the formation of the vascular system27. A genetic defect of endoglin causes the underdevelopment of vascular systems28. In several malignancies, high levels of endoglin expression are frequently observed and correlated with poor prognosis13. In our study, the expression of endoglin was significantly higher in the advanced stage group than in the early stage group.(Table 2, Figs. 2. A, 2. B) This suggests that a well-developed micro-vessel is a prerequisite for OSCC growth. These results are consistent with those of other studies. Schimming and colleagues29,30 reported that the expression of endoglin was significantly correlated with the TNM and T stages in OSCC patients. In terms of N stage, our studies showed no correlation between the expression of endoglin and lymph node metastasis. Nonetheless, other studies reported that the expression of endoglin is closely correlated with lymph node metastasis in OSCC15,31,32. These conflicting results might be due to the small number of samples in our study.
Podoplanin is a transmembrane glycoprotein that is mostly observed in lymphatic endothelial cells and not in vascular endothelial cells. Thus, recently, podoplanin antibodies have been widely used to detect lymphatic vessels in several malignancies14,33. For OSCC, Funayama et al.34 reported that podoplanin was expressed less in normal tissues than in malignant tissues. The closer the pre-malignant lesion to the malignant lesion is, the higher the expression of podoplanin34,35. Even though there is some controversy36, the expression of podoplanin is closely correlated with regional lymph node metastasis and poor prognosis in OSCC37-43. In our results, there was no significant correlation between the expression of podoplanin and either the tumor stage or lymph node metastasis. Note, however, that the average expression level of podoplanin in the lymph node positive group was higher than that of the lymph node negative group.(Table 2)
In this study, micro-vessels and lymphatic vessels were successfully distinguished by endoglin and podoplanin antibodies. In most serial sectioned images, the endothelial cell, which is positive to both antibodies, could not be found.(Figs. 1. A, 1. B) These findings are similar to the results of another study that used double immunohistochemical staining to detect micro and lymphatic vessels15. Endoglin was only positive for vascular endothelial cells (Fig. 1. A), whereas podoplanin was positive not only for endothelial cells in the lymphatic vessels but also for basal cells in the basal layer of the mucosa.(Fig. 1. C) Podoplanin-positive basal cells can affect the computer-assisted detection of lymphatic endothelial cells. Kanner et al.44 reported that podoplanin is a novel marker for detecting lymphatic endothelial cells. Note, however, that the density of the lymphatic vessels measured by podoplanin could be misinterpreted because the basal cells of the skin or mucosa also have reactivity to podoplanin. Thus, in this study, for the analysis of only lymphatic endothelial cells, areas that included the podoplanin-positive basal cells were deleted by an image editing process prior to the analysis.
Micro-vessels and lymphatic vessels detected by endoglin and podoplanin antibodies were observed as an oval-shaped ring.(Fig. 1) Various sizes and shapes of vessels were observed in these images. This is because each vessel has originally various shapes and sizes and multidirectional cross sections due to the different planes of the slice. In our study, computer-assisted image analysis was done to evaluate the expression of vascular or lymphatic endothelial cells with several clinical parameters11,45,46. This method might be more accurate than counting the numbers of vascular or lymphatic vessels in specific regions.
This study had a small sample size compared with those of other studies15,29,31. In our results, though, a high expression of endoglin was significantly correlated with advanced-stage OSCC. For clinical application, further studies on the correlation between the expression of endoglin and prognosis-related factors such as survival rate and metastasis in a larger sample population are needed. If our results could be supported by these further studies, analysis of the endoglin expression in the biopsied tissues could be helpful to clinicians and patients in evaluating the prognosis of OSCC.
Figures and Tables
Fig. 1
A, B. Micro-vessels (A) and lymphatic vessels (B) are, for the most part, successfully distinguished by endoglin and podoplanin in serial sections. C. Podoplanin is expressed not only by the membrane of lymphatic endothelial cells but also by the membrane of basal cells (A-C: immunohistochemical (IHC) staining, ×400). D. The expression of endoglin was measured by the numbers of pixels which appeared as red using image analysis software (D: IHC staining, ×100).
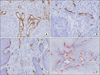
Fig. 2
A, B. Representative endoglin expression in advanced stage (A) and in early stage (B) oral squamous cell carcinoma (immunohistochemical (IHC) staining, ×100). C, D. Representative podoplanin expression in advanced stage (C) and in early stage (D) oral squamous cell carcinoma (IHC staining, ×100).
References
1. McDowell JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am. 2006. 39:277–294.

2. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncol. 2000. 36:311–327.

3. Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000. 36:256–263.

4. Oliveira LR, Ribeiro-Silva A. Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2011. 40:298–307.

5. Scully C, Bagan JV. Recent advances in oral oncology 2008; squamous cell carcinoma imaging, treatment, prognostication and treatment outcomes. Oral Oncol. 2009. 45:e25–e30.

6. Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:67–76.

7. van der Schroeff MP, Baatenburg de Jong RJ. Staging and prognosis in head and neck cancer. Oral Oncol. 2009. 45:356–360.

8. Kim JY, Rotaru H, Kim SG. The clinical significance of the expression of TGF-beta 1 and MMP-2 related to the regional lymph node metastasis in the oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg. 2007. 33:199–203.
11. Palomba A, Gallo O, Brahimi A, Franchi A. Evaluation of lymphangiogenesis in premalignant conditions of the head and neck mucosa. Head Neck. 2010. 32:1681–1685.

12. Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009. 13:1405–1416.

13. Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003. 17:984–992.

14. Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008. 28:2997–3006.
15. Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as a prognostic factor in head and neck squamous cell carcinoma. Virchows Arch. 2006. 448:768–775.

16. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.

17. Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer--a review of mechanisms. Int J Oral Sci. 2010. 2:5–14.

19. Tortora G, Melisi D, Ciardiello F. Angiogenesis: a target for cancer therapy. Curr Pharm Des. 2004. 10:11–26.

20. Dredge K, Dalgleish AG, Marriott JB. Angiogenesis inhibitors in cancer therapy. Curr Opin Investig Drugs. 2003. 4:667–674.
22. Rugo HS. Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist. 2004. 9:Suppl 1. 43–49.

23. Planchard D. Bevacizumab in non-small-cell lung cancer: a review. Expert Rev Anticancer Ther. 2011. 11:1163–1179.

24. Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess. 2007. 11:1–128.

25. Lastres P, Letamendía A, Zhang H, Rius C, Almendro N, Raab U, et al. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996. 133:1109–1121.

26. Quintanilla M, Ramirez JR, Pérez-Gómez E, Romero D, Velasco B, Letarte M, et al. Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene. 2003. 22:5976–5985.

27. Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000. 217:42–53.

28. Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999. 284:1534–1537.

29. Schimming R, Reusch P, Kuschnierz J, Schmelzeisen R. Angiogenic factors in squamous cell carcinoma of the oral cavity: do they have prognostic relevance? J Craniomaxillofac Surg. 2004. 32:176–181.

30. Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002. 24:151–156.

31. Eshghyar N, Mohammadi N, Rahrotaban S, Motahhary P, Vahedi Vaez SM. Endoglin (CD105) positive microvessel density and its relationship with lymph node metastasis in squamous cell carcinoma of the tongue. Arch Iran Med. 2011. 14:276–280.
32. Chuang HC, Su CY, Huang HY, Chien CY, Chen CM, Huang CC. High expression of CD105 as a prognostic predictor of early tongue cancer. Laryngoscope. 2006. 116:1175–1179.

33. Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007. 96:1–5.

34. Funayama A, Cheng J, Maruyama S, Yamazaki M, Kobayashi T, Syafriadi M, et al. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011. 78:171–180.

35. Siriwardena BS, Kudo Y, Ogawa I, Udagama MN, Tilakaratne WM, Takata T. VEGF-C is associated with lymphatic status and invasion in oral cancer. J Clin Pathol. 2008. 61:103–108.

36. Zhang Z, Pan J, Li L, Wang Z, Xiao W, Li N. Survey of risk factors contributed to lymphatic metastasis in patients with oral tongue cancer by immunohistochemistry. J Oral Pathol Med. 2011. 40:127–134.

37. Okada Y. Relationships of cervical lymph node metastasis to histopathological malignancy grade, tumor angiogenesis, and lymphatic invasion in tongue cancer. Odontology. 2010. 98:153–159.

38. Zhao D, Pan J, Li XQ, Wang XY, Tang C, Xuan M. Intratumoral lymphangiogenesis in oral squamous cell carcinoma and its clinicopathological significance. J Oral Pathol Med. 2008. 37:616–625.

39. Longatto Filho A, Oliveira TG, Pinheiro C, de Carvalho MB, Curioni OA, Mercante AM, et al. How useful is the assessment of lymphatic vascular density in oral carcinoma prognosis? World J Surg Oncol. 2007. 5:140.

40. Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007. 110:1287–1294.

41. Kreppel M, Scheer M, Drebber U, Ritter L, Zöller JE. Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010. 456:473–482.

42. Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006. 107:563–569.

43. Huber GF, Fritzsche FR, Züllig L, Storz M, Graf N, Haerle SK, et al. Podoplanin expression correlates with sentinel lymph node metastasis in early squamous cell carcinomas of the oral cavity and oropharynx. Int J Cancer. 2011. 129:1404–1409.

44. Kanner WA, Galgano MT, Atkins KA. Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. Appl Immunohistochem Mol Morphol. 2010. 18:226–230.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download