Abstract
Pancreatic islets are responsible for blood glucose homeostasis. Reduced numbers of functional (insulin-secreting) beta-cells in pancreatic islets underlies diabetes. Restoration of the secretion of the proper amount of insulin is a goal. Beta-cell mass is increased by neogenesis, proliferation and cell hypertrophy, and is decreased by beta-cell death primarily through apoptosis. Many hormones and nutrients affect beta-cell mass, and glucose and free fatty acid are thought to be the most important determinants of beta-cell equilibrium. A number of molecular pathways have been implicated in beta-cell mass regulation and have been studied. This review will focus on the role of the principle metabolites, glucose and free fatty acid, and the downstream signaling pathways regulating beta-cell mass by these metabolites.
Beta-cell death is a major pathogenic component in diabetes. Type 1 diabetes results from an absolute insulin deficiency due to autoimmune-related beta-cell death. Type 2 diabetes is a condition of relative insulin deficiency as a result of beta-cell dysfunction and death as the combined consequence of increased circulating glucose and saturated fatty acids [1]. Control of blood glucose levels depends on changes in insulin production and secretion by the pancreatic beta-cells. So, it is critically important to maintain an adequate beta-cell mass in response to various changes. During adult life, beta-cell mass may have to adapt in response to increased demands due to increased body mass, pregnancy, or even loss of insulin sensitivity of peripheral tissues [2]. If such compensatory adaptation is inadequate, glucose homeostasis will be compromised, resulting in chronically elevated blood glucose or diabetes. In insulin-resistant, 12-week-old db/db mice, increased beta-cell mass is seen compared with 4-week-old lean control mice (Fig. 1), which is believed to compensate for an increased insulin demand. Failure to compensate leads to decreased beta cell mass later in life (as shown in 24-week-old mice). The affected db/db mice eventually become diabetic, more obese and glucose intolerant.
Beta-cell mass regulation is modulated by various environmental factors and nutrients including glucose, insulin, amino acids, fatty acids and various other growth factors/hormones including insulin like growth factor-I, glucagon-like peptide-1 and betacellulin [3]. These growth factors and nutrients can affect a variety of beta-cell functions, and can suppress or stimulate beta-cell replication, survival and mass expansion through different intracellular pathways.
Glucose and free fatty acids (FFA) are two main nutrients in energy metabolism in most organisms and are of particular interest in beta-cell equilibrium. Acute elevated glucose and fatty acid levels increased beta-cell viability and function, but glucotoxicity and lipotoxicity caused by chronic hyperglycemia and dyslipidemia impairs the metabolism of glucose and lipids in a detrimental cycle leading to further beta-cell damage. This review briefly outlines the significant factors regulating beta-cell mass and their signal transduction pathway, with focus on postnatal regulation of glucose and FFA (Table 1) [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53].
Glucose is thought to be the most important determinant of beta-cell mass equilibrium [54]. Glucose is the pathological hallmark of diabetes and is a potential contributor to further decline in beta-cell mass through what has been termed glucotoxicity.
Neonatal beta-cells, insulinoma cell lines and primary islets have a growth advantage in response to glucose. Glucose infusion (22 mM) for a short time (24-48 hours) in normal rats and rats in whom diabetes has been induced using streptozotocin induces beta-cell mass, due mainly to the rapid activation of neogenesis of new endocrine cells and suppression of beta-cell apoptosis [55]. Glucose infusion for 48 hours reportedly results in increased beta-cell replication and hypertrophy, and leads to sustained effects on beta-cell mass after glucose infusion is stopped [56]. The same results were reported using human islets. Short term (1-3 days) exposure of cultured human islets to 33.3 mM glucose increased the number of proliferating (Ki-67 positive cells) beta-cells, whereas prolonged exposure resulted in an inhibition of proliferation, relative to islets at 5.5 mM glucose [57].
Glucose influences extracellular mitogens, internal receptor-related signaling, downstream signaling pathways and glucose metabolic pathways. The elucidation of glucose-mediated growth-regulating pathways may reveal novel pharmacological means of expanding the remaining endogenous beta-cell population in diabetes.
The mitogenic effect of glucose is associated with insulin secretion that is potentially induced by glucose concentration. Insulin is a robust mitogen for some cell types, and insulin signaling is involved in beta-cell replication [4]. In vitro, glucose modulates intracellular signaling molecules of the insulin signaling pathway including insulin receptor 2 (IRS-2), phosphatidylinositol 3-kinase (PI3K), protein kinase B (PKB), glycogen synthase kinase (GSK-3), extracellular signaling-related kinase (ERK1/2), and mammalian target of rapamycin (mTOR). Among them, IRS-2 is a central factor that mediates signals from several known beta-cell glucose mitogens. Mice lacking IRS-2 display increased insulin resistance in liver tissue, but beta-cells are unable to effectively compensate by insulin stimulation. In these mice, beta cell proliferation is reduced, beta-cell mass declines and the mice become diabetic [5]. Assmann et al. [6] reported that beta-cells from IRS-2 KO mice display reduced DNA synthesis in response to glucose stimulation in vitro. These results suggest that IRS-2 is essential for beta-cell compensation for increased insulin demand due to peripheral insulin resistance. In contrast, increased IRS-2 expression promotes beta-cell replication and prevents diabetes [7]. In IRS-2 overexpressing insulinoma cells, glucose-induced beta-cell proliferation is synergistic with IRS-2 [8]. Down-stream signaling activation of PI3K/PKB, GSK-3, ERK, and mTOR are also major effectors of beta cell proliferation. Inhibition of mTOR with rapamycin and PI3K with wortmannin blocks glucose-stimulated beta-cell proliferation in INS-1 cells and rat islets, respectively [9, 10]. Glucose mediated inactivation of forkhead box O (FoxO) transcription factors, which are PI3K/PKB targets, lead to down-regulation of the transcriptional repressor B-cell lymphoma 6 (BCL-6), increased cyclin D2 expression and increased beta-cell proliferation [11].
The mitogen effect of glucose on beta-cells is mediated by glucokinase (GK), an enzyme involved in glucose metabolism (glycolysis) in beta-cells. GK phosphorylates glucose to glucose-6-phosphate and is the glucose sensor that regulates insulin secretion in beta-cells [12]. When fed a high-fat diet, GK+/- mice display decreased beta-cell replication and insufficient beta-cell hyperplasia. Islets from GK+/- mice are reportedly reduced in their expression of IRS-2 compared with high-fat fed wild-type mice [13]. These results suggest that GK is critical for beta-cell hyperplasia in response to insulin resistance. Recently, the effect of various GK activators including YH-GKA, Compound A, GKA50, and LY2121260 that trigger GK by binding to an allosteric site of the enzyme have been tested for their capacity to induce beta-cell proliferation in vitro and in vivo [14, 15]. We have reported that administration of YH-GKA lowers glucose levels in db/db mice [16], increases beta-cell growth and prevents glucotoxic beta-cell apoptosis in INS-1 cells. We also demonstrated that YH-GKA also induces concomitant up-regulation of IRS-2 and activation of the AKT-mediated GSK-3/beta-catenin pathway [17]. Failure of beta-cell adaptation has been correlated with reduced activities of pyruvate carboxylase (PC) and pyruvate dehydrogenase, which catalyze pyruvate conversion to the Krebs cycle intermediates oxaloacetate and acetyl-CoA [18]. Islet PC activity and protein are reportedly reduced in Agouti-K (AyK) mice that are severely hyperglycemic compared with less severely affected AyK mice [19]. The PC inhibitor phenylacetic acid decreases beta-cell proliferation in 60% pancreatectomized rats and in Zucker diabetic fatty (ZDF) rats [20], indicating the importance of this enzyme in the adaptive beta-cell response to both insulin resistance and diminished beta cell mass in vivo.
The level and duration of hyperglycemia are crucial in determining the fate of beta-cells. Prolonged hyperglycemia appears to have a proapoptotic effect, which is referred to as glucose toxicity. Glucotoxicity refers to the slow and irreversible detrimental effects of chronically elevated glucose levels on beta-cell function, characterized by decreased insulin synthesis caused by reduced insulin gene expression [28]. A number of stress-related mechanisms have been proposed to explain how chronically elevated glucose levels impair beta-cell function and increase beta-cell apoptosis rates.
The endoplasmic reticulum (ER) is responsible for the synthesis of all secreted proteins including insulin, the most abundant protein produced by beta-cells. A sustained increased demand for insulin due to chronic hyperglycaemia may impose a stress on the ER. High glucose levels induce components of ER stress responses, such as x-box protein 1 (XBP-1), inositol requiring enzyme 1 (IRE1), and CCAAT-enhancer-binding protein (C/EBP) homologous protein (CHOP) in beta-cell lines and isolated islets from rat, mouse, and humans [21, 22]. Beta-cell dysfunction is one of the important factors that ER stress mediated beta-cell apoptosis [23, 24]. ER stress mediated beta-cell dysfunction may be important in the transition from pre-diabetes to diabetes, because this further decline in cell mass is probably not the cause of diabetes in the absence of beta-cell dysfunction [25]. When patients have lost most of their beta-cells, increased workload for production of insulin on the remaining beta-cells might cause chronic ER stress, leading to translational attenuation of proinsulin and degradation of insulin mRNA. This adaptive response exacerbates chronic hyperglycemia, leading to ER stress and ER stress-mediated beta-cell apoptosis. Discovering methods that could reduce ER stress to a tolerable state could lead to novel and efficient therapeutic treatments for diabetes [26, 27].
Elevated glucose levels augment the generation of reactive oxygen species (ROS) in islet cells, which induce oxidative stress. ROS is produced following oxidative phosphorylation of glucose in mitochondria. Since beta-cells have very low levels of antioxidant enzymes, they are particularly vulnerable to oxidative stress [28]. Isolated islets from type 2 diabetics can display increased content of markers of oxidative stress, such as nitrotyrosine and 8-hydroxy-2-deoxyguanosine, compared with healthy controls, and oxidative stress correlates with the degree of impairment in insulin secretion [29]. Anti-oxidants including N-acetyl-cysteine and aminoguanidine can protect HIT-T15 cells and isolated islets during prolonged culture in the presence of elevated glucose (27-38 mM glucose) [30] and N-acetyl-cysteine treatment of ZDF rats and db/db mice results in decreased production of markers for oxidative stress, improved insulin gene expression and improved glycemic control [28]. Increased glucose and ROS levels activate uncoupling protein 2 (UCP2), which reduces mitochondrial membrane potential with concurrent production of heat. Although uncoupling of oxidative phosphorylation may protect from additional ROS production and oxidative stress in beta-cells, adenosine triphosphate synthesis, which is necessary for insulin secretion, is reduced. Therefore, increased UCP2 expression may contribute to the harmful effects on beta-cell function [31].
Pro-inflammatory cytokines like interleukin 1β (IL-1β) and nuclear factor κB (NF-κB) may be involved in autoimmune reactions leading to beta-cell apoptosis in type 2 diabetes. Prolonged exposure of human islets to hyperglycemia reportedly triggers the production of IL-1β and NF-κB by beta-cells, leading to autocrine apoptosis [32]. This result and its implications remain debatable. Elevated glucose did not increase the mRNA levels of the NF-κB target genes, such as inducible nitric oxide synthase, NF-κB inhibitor alpha (IκBα) and pro-IL1β, in cultured rat and human islets in one study [33]. Still, the beneficial effect of the recombinant IL-1 receptor antagonist anakinra on glucose tolerance in human type 2 diabetics indicates that circulating or locally produced IL-1β contributes to glucose intolerance [34].
Another proposed stimulus for beta-cell mass regulation is FFA. Acute exposure to FFA stimulates insulin secretion and beta-cell proliferation, while prolonged exposure to FFA decreases glucose-stimulated insulin secretion and induces insulin resistance and beta-cell dysfunction in both animal models and humans [58].
FFA have been postulated to promote compensatory beta-cell proliferation [59]. Treatment with FFA increases beta-cell proliferation in rat islets [60, 61] and intralipid infusion into normal rat increases beta-cell mass and proliferation [62, 63]. However, whether in vivo exposure to lipid promotes beta-cell proliferation remains questionable and how this relates to human biology is unclear. FFA are also important for normal beta-cell function, particularly the compensation for insulin resistance. Acute FFA exposure enhances glucose and non-glucose stimulated insulin secretion in vitro [64]. For example, infusion of 0.5 mM palmitate during food deprivation in normal rats results in supranormal glucose-stimulated insulin secretion and 24-hour intralipid (10% triglyceride) infusion into healthy subjects also significantly increases insulin response [65].
FFA and other lipid molecules are important for many cellular functions including vesicle exocytosis. The presence of some FFA is essential for glucose-stimulated insulin secretion, which is of particular relevance for beta-cell compensation for insulin resistance.
An insulinotropic effect of FFA via beta-cell lipid signaling pathways has been proposed [35]. In this scenario, FFA and other nutrient secretagogues contribute analplerosis, which follows cataplerotic efflux of citrate from mitochondria. This pathway induces malonyl-CoA formation, carnitine palmitoyltransferase 1 activity and fatty acid oxidation inhibition, which lead to the accumulation of long-chain acyl-CoAs that stimulate insulin secretion directly or by the formation of complex lipids like diacylglycerol and various phospholipids. The second pathway involves cycling of triglyceride and FFA, which promotes fatty acid esterification and lipolysis. In the presence of exogenous FFA, cycle intermediates, such as long-chain acyl-CoAs, diacylglycerol, phospholipid and FFA, accumulate and may modulate insulin secretion.
FFA activate G-protein coupled receptor 40 (also known as FFAR1), which are expressed on the surface of human and rodent beta-cells. This signaling mediates positive effects including insulin secretion [36]. Saturated FA decreases FFAR1 expression, while unsaturated FA increases increases FFAR1 expression and this induction protects against lipotoxicity in INS-1 cells [37]. A single nucleotide polymorphism at the FFAR1 locus has been correlated with insulin secretory function in humans [38]. FFAR1 agonists including Tak-875 and Gw-9508 potentiate glucose-stimulated insulin secretion in diabetic ZDF rats, suggesting that FFAR1 agonists might be a useful therapy for type 2 diabetes [39]. In contrast, FFAR signaling may be related with lipotoxic effects. Palmitate treatment of human islets was reported to decrease insulin contents and secretion, both of which were prevented by FFAR1 antagonists [40].
Prolonged in vitro exposure of isolated islets or insulin-secreting cell lines to elevated levels of fatty acids is associated with inhibition of glucose-induced insulin secretion, impairment of insulin gene expression and induction of cell death by apoptosis. Treatment of islets with 1 mM FFA (plasma levels of prediabetic and diabetic animals) leads to a significant increase in DNA laddering and ceramide formation compared with non-treated islets [66]. Moreover 48-hour treatment of islets of normal rats with 0.125-0.5 mM palmitate inhibits insulin secretion stimulated by 27 mM glucose [67]. Importantly, in vitro [68] and in vivo [69] studies have provided evidence that lipotoxicity is synergistically increased in the presence of concomitantly elevated glucose levels [70].
Chronic exposure of pancreatic beta-cells to FFA elicits multiple mechanisms of toxicity, including accelerated ceramide synthesis, activation of ER stress and oxidative stress. Rat islets cultured for 7 days in the presence of elevated concentrations of FFA exhibit changes in apoptosis, DNA fragmentation, caspase 3 activity and expression of apoptotic genes, similar to those seen with chronic exposure to elevated levels of glucose.
Palmitate induces ceramide accumulation by a dual mechanism involving serine palmitoyl-trasnferase and the formation of ceramides with specific N-acyl chain lengths by ceramide synthase 4 [35]. Accumulating evidence indicates that the apoptosis of pancreatic beta cells has a direct relationship with ceramide formation. An elevated level of ceramide has been reported in the islets of ZDF rats and beta-cell apoptosis mediated by the enhanced ceramide biosynthesis can be effectively prevented by treatment with fumonisin B1, a ceramide synthase inhibitor [41]. Moreover, C2-ceramide, an analog of ceramide that can cross cell membranes, is able to potentiate the effects of palmtate on pro-apoptosis and anti-proliferation in beta-cells [42].
ER stress has been linked to apoptosis in beta-cells chronically exposed to elevated levels of fatty acid [43, 44], with ER stress markers being elevated in pancreatic islets of patients with diabetes [21]. The detailed mechanism of generation of ER stress induced by saturated fatty acids, such as palmitate, in beta-cells mainly involves the loss of calcium ion and protein overload. The activity of ER Ca2+ channels regulates the susceptibility of beta-cells to ER stress and palmitate is more effective at lowering ER Ca2+ than cytokines or glucotoxicity [45]. Lipotoxicity has disrupts ER-to-Golgi protein trafficking due to protein overload, resulting in impaired pro-insulin maturation and loss of insulin content before apoptosis [46]. Lipotoxicity can induce ER stress-triggered apoptosis in several ways. CHOP and activating transcription factor 4 (ATF-4) are well-known transcription factors induced by lipotoxic stress that has been directly linked to the intrinsic apoptotic pathway [43]. Moreover, early translational repression via pancreatic ER kinase/eukaryotic translation initiation factor 2α subunit phosphorylation leads to loss of the myeloid cell leukemia sequence 1 protein, which is an anti-apoptotic member of the BH3 family [47]. Autophagy is triggered by ER stress in various cell types, indicating a correlation between ER stress and autophagy induced by lipotoxocity. Autophagy in response to fatty acid treatment can be partially inhibited with the protein folding chaperone, 4-phenylbutyrate, and ER stress markers are reportedly reduced in autophagy-deficient islets [48, 49]. Contrarily, fatty acid inhibition of autophagy in beta-cells has been described [50]. Further studies are needed to sort out this dichotomy.
Increased ROS levels are the important trigger for beta-cell dysfunction. Glucose-stimulated insulin secretion is decreased in MIN-6 cells and rat islets exposed to FFA for 48 hours. This decrease can be obviated by the antioxidant taurine. N-acetyl cysteine and tempol also prevent the impairment in beta-cell function induced by FFA in vivo during hyperglycemic clamping and ex vivo in isolated islets of oleate treated rats [51]. The detailed molecular mechanisms of ROS-mediated lipoxotivity have been analyzed using RINm5F and INS-1 cells, as well as primary islets; only long-chain (>C14) saturated nonesterified fatty acid were toxic to beta-cells [52]. Moreover, overexpression of catalase in peroxisomes and in the cytosol, but not in mitochondria, can reduce H2O2 formation and protect cells from palmitate-induced toxicity, demonstrating that formation of hydrogen peroxide in peroxisomes is responsible for long chain fatty acid-induced toxicity [52, 53].
The balance between apoptosis and replication seems to be pivotal in beta-cell mass maintenance. Failure in this balance results in beta-cell dysfunction and diabetes onset. The available data indicate that beta-cell mass changes in response to metabolic demand and that this process is regulated by different nutrients. Glucose and FFA may have inhibitory and stimulating effects on beta-cell apoptosis. However, both the level and duration of hyperglycaemia/hyperlipidemia appear crucial in determining the fate of beta-cells. Identification of the molecular mechanisms of beta-cell mass regulation and a better understanding of the processes of proliferation should provide further guidance in the development of new therapeutic targets for diabetes.
Figures and Tables
Fig. 1
Beta-cell mass regulation in db/db mice. Images of islet morphology at 4, 12, and 24 weeks of age in db/db mice. Sections were immunohistochemically stained for insulin and glucagon as a measurement of beta-cells (green) and alpha-cells (red). Scale bars=20 µm.
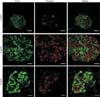
Table 1
Influence of glucose and free fatty acids on beta-cell mass regulation

| Nutrients | Impact on beta cell mass regulation | Possible mechanisms |
|---|---|---|
| Glucose | Stimulatory effects | Insulin signaling [4, 5, 6, 7, 8, 9, 10, 11] |
| Glucose metabolic pathways [12, 13, 14, 15, 16, 17, 18, 19, 20] | ||
| Inhibitory effects | ER stress [21, 22, 23, 24, 25, 26, 27] | |
| Oxidative stress and mitochondrial dysfunction [28, 29, 30, 31] | ||
| Inflammation [32,33,34] | ||
| Hypoxia | ||
| Free fatty acids | Stimulatory effects | Lipid signaling metabolite and GPR signaling [35, 36, 37, 38, 39, 40] |
| Cell cycle regulators | ||
| Inhibitory effects | Formation of ceramide [35, 41, 42] | |
| ER stress and autophagy [43, 44, 45, 46, 47, 48, 49, 50] | ||
| Oxidative stress [51, 52, 53] | ||
| Inflammation |
Acknowledgements
This study was supported by the grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (No. 2010-0009378).
References
1. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005; 54:Suppl 2. S97–S107.
2. Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001; 414:792–798.
3. Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001; 50:Suppl 1. S25–S29.
4. Movassat J, Saulnier C, Portha B. Insulin administration enhances growth of the beta-cell mass in streptozotocin-treated newborn rats. Diabetes. 1997; 46:1445–1452.
5. Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000; 49:1880–1889.
6. Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009; 29:3219–3228.
7. Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest. 2003; 112:1521–1532.
8. Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes. 2002; 51:966–976.
9. Hugl SR, White MF, Rhodes CJ. Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem. 1998; 273:17771–17779.
10. Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004; 53:Suppl 3. S225–S232.
11. Glauser DA, Schlegel W. The FoxO/Bcl-6/cyclin D2 pathway mediates metabolic and growth factor stimulation of proliferation in Min6 pancreatic beta-cells. J Recept Signal Transduct Res. 2009; 29:293–298.
12. Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009; 8:399–416.
13. Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007; 117:246–257.
14. Wei P, Shi M, Barnum S, Cho H, Carlson T, Fraser JD. Effects of glucokinase activators GKA50 and LY2121260 on proliferation and apoptosis in pancreatic INS-1 beta cells. Diabetologia. 2009; 52:2142–2150.
15. Nakamura A, Terauchi Y, Ohyama S, Kubota J, Shimazaki H, Nambu T, Takamoto I, Kubota N, Eiki J, Yoshioka N, Kadowaki T, Koike T. Impact of small-molecule glucokinase activator on glucose metabolism and beta-cell mass. Endocrinology. 2009; 150:1147–1154.
16. Park K. Identification of YH-GKA, a novel benzamide glucokinase activator as therapeutic candidate for type 2 diabetes mellitus. Arch Pharm Res. 2012; 35:2029–2033.
17. Oh YS, Lee YJ, Park K, Choi HH, Yoo S, Jun HS. Treatment with glucokinase activator, YH-GKA, increases cell proliferation and decreases glucotoxic apoptosis in INS-1 cells. Eur J Pharm Sci. 2014; 51:137–145.
18. MacDonald MJ, Tang J, Polonsky KS. Low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of Zucker diabetic fatty rats. Diabetes. 1996; 45:1626–1630.
19. Han J, Liu YQ. Reduction of islet pyruvate carboxylase activity might be related to the development of type 2 diabetes mellitus in Agouti-K mice. J Endocrinol. 2010; 204:143–152.
20. Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002; 277:39163–39168.
21. Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007; 50:2486–2494.
22. Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006; 4:245–254.
23. Liew CW, Bochenski J, Kawamori D, Hu J, Leech CA, Wanic K, Malecki M, Warram JH, Qi L, Krolewski AS, Kulkarni RN. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010; 120:2876–2888.
24. Wali JA, Rondas D, McKenzie MD, Zhao Y, Elkerbout L, Fynch S, Gurzov EN, Akira S, Mathieu C, Kay TW, Overbergh L, Strasser A, Thomas HE. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell Death Dis. 2014; 5:e1124.
25. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab. 2011; 22:266–274.
26. Oh YS, Lee YJ, Kang Y, Han J, Lim OK, Jun HS. Exendin-4 inhibits glucolipotoxic ER stress in pancreatic beta cells via regulation of SREBP1c and C/EBPbeta transcription factors. J Endocrinol. 2013; 216:343–352.
27. Madec AM, Cassel R, Dubois S, Ducreux S, Vial G, Chauvin MA, Mesnier A, Chikh K, Bosco D, Rieusset J, Van Coppenolle F, Thivolet C. Losartan, an angiotensin II type 1 receptor blocker, protects human islets from glucotoxicity through the phospholipase C pathway. FASEB J. 2013; 27:5122–5130.
28. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008; 29:351–366.
29. Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005; 54:727–735.
30. Tajiri Y, Moller C, Grill V. Long-term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology. 1997; 138:273–280.
31. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006; 116:1802–1812.
32. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002; 110:851–860.
33. Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes. 2005; 54:3238–3244.
34. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007; 356:1517–1526.
35. Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006; 55:Suppl 2. S16–S23.
36. Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: how much do we know? Trends Endocrinol Metab. 2013; 24:398–407.
37. Tuo Y, Feng DD, Wang DF, Sun J, Li SB, Chen C. Long-term in vitro treatment of INS-1 rat pancreatic beta-cells by unsaturated free fatty acids protects cells against gluco- and lipotoxicities via activation of GPR40 receptors. Clin Exp Pharmacol Physiol. 2012; 39:423–428.
38. Wagner R, Kaiser G, Gerst F, Christiansen E, Due-Hansen ME, Grundmann M, Machicao F, Peter A, Kostenis E, Ulven T, Fritsche A, Häring HU, Ullrich S. Reevaluation of fatty acid receptor 1 as a drug target for the stimulation of insulin secretion in humans. Diabetes. 2013; 62:2106–2111.
39. Poitout V, Lin DC. Modulating GPR40: therapeutic promise and potential in diabetes. Drug Discov Today. 2013; 18:1301–1308.
40. Kristinsson H, Smith DM, Bergsten P, Sargsyan E. FFAR1 is involved in both the acute and chronic effects of palmitate on insulin secretion. Endocrinology. 2013; 154:4078–4088.
41. Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998; 273:32487–32490.
42. Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003; 52:726–733.
43. Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab. 2014; 25:389–398.
44. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007; 50:752–763.
45. Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology. 2014; 155:758–768.
46. Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009; 52:2369–2373.
47. Allagnat F, Cunha D, Moore F, Vanderwinden JM, Eizirik DL, Cardozo AK. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to beta-cell apoptosis. Cell Death Differ. 2011; 18:328–337.
48. Choi SE, Lee SM, Lee YJ, Li LJ, Lee SJ, Lee JH, Kim Y, Jun HS, Lee KW, Kang Y. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 2009; 150:126–134.
49. Quan W, Hur KY, Lim Y, Oh SH, Lee JC, Kim KH, Kim GH, Kim SW, Kim HL, Lee MK, Kim KW, Kim J, Komatsu M, Lee MS. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012; 55:392–403.
50. Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in beta-cells. J Biol Chem. 2011; 286:42534–42544.
51. Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007; 56:2927–2937.
52. Elsner M, Gehrmann W, Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011; 60:200–208.
53. Gehrmann W, Elsner M, Lenzen S. Role of metabolically generated reactive oxygen species for lipotoxicity in pancreatic beta-cells. Diabetes Obes Metab. 2010; 12:Suppl 2. 149–158.
54. Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009; 11:Suppl 4. 65–81.
55. Bernard C, Berthault MF, Saulnier C, Ktorza A. Neogenesis vs. apoptosis As main components of pancreatic beta cell ass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 1999; 13:1195–1205.
56. Topp BG, McArthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats. Diabetologia. 2004; 47:1602–1610.
57. Maedler K, Schumann DM, Schulthess F, Oberholzer J, Bosco D, Berney T, Donath MY. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006; 55:2455–2462.
58. Sharma RB, Alonso LC. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014; 14:492.
59. Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008; 29:647–676.
60. Brelje TC, Bhagroo NV, Stout LE, Sorenson RL. Beneficial effects of lipids and prolactin on insulin secretion and beta-cell proliferation: a role for lipids in the adaptation of islets to pregnancy. J Endocrinol. 2008; 197:265–276.
61. Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, McDaniel ML, Greenberg AS, Kwon G. Beta-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets. 2012; 4:379–392.
62. Fontes G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TL, Poitout V. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia. 2010; 53:2369–2379.
63. Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001; 280:E788–E796.
64. Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996; 97:2728–2735.
65. Paolisso G, Gambardella A, Amato L, Tortoriello R, D'Amore A, Varricchio M, D'Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995; 38:1295–1299.
66. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998; 95:2498–2502.
67. Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994; 93:870–876.
68. Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001; 50:315–321.
69. Briaud I, Kelpe CL, Johnson LM, Tran PO, Poitout V. Differential effects of hyperlipidemia on insulin secretion in islets of langerhans from hyperglycemic versus normoglycemic rats. Diabetes. 2002; 51:662–668.
70. El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003; 144:4154–4163.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download