1. Holmlund A, Hedin M, Pussinen PJ, Lerner UH, Lind L. Porphyromonas gingivalis (Pg) a possible link between impaired oral health and acute myocardial infarction. Int J Cardiol. 2011; 148:148–153.

2. Glurich I, Grossi S, Albini B, Ho A, Shah R, Zeid M, et al. Systemic inflammation in cardiovascular and periodontal disease: comparative study. Clin Diagn Lab Immunol. 2002; 9:425–432.

3. Persson GR, Persson RE. Cardiovascular disease and periodontitis: an update on the associations and risk. J Clin Periodontol. 2008; 35:8 Suppl. 362–379.

4. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–1812.

5. Pitiphat W, Savetsilp W, Wara-Aswapati N. C-reactive protein associated with periodontitis in a Thai population. J Clin Periodontol. 2008; 35:120–125.

6. Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: the immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005; 46:389–403.

7. Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999; 37:381–388.

8. Cunnane G, Grehan S, Geoghegan S, McCormack C, Shields D, Whitehead AS, et al. Serum amyloid A in the assessment of early inflammatory arthritis. J Rheumatol. 2000; 27:58–63.
9. Mayer JM, Raraty M, Slavin J, Kemppainen E, Fitzpatrick J, Hietaranta A, et al. Serum amyloid A is a better early predictor of severity than C-reactive protein in acute pancreatitis. Br J Surg. 2002; 89:163–171.

10. Rivera MF, Lee JY, Aneja M, Goswami V, Liu L, Velsko IM, et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One. 2013; 8:e57178.

11. Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000; 27:537–541.

12. Dye BA, Choudhary K, Shea S, Papapanou PN. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J Clin Periodontol. 2005; 32:1189–1199.

13. Aoki-Nonaka Y, Nakajima T, Miyauchi S, Miyazawa H, Yamada H, Domon H, et al. Natural killer T cells mediate alveolar bone resorption and a systemic inflammatory response in response to oral infection of mice with Porphyromonas gingivalis. J Periodontal Res. 2014; 49:69–76.

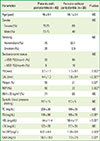
14. Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012; 83:1449–1454.

15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
16. Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001; 358:24–29.

17. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107:499–511.
18. Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996; 11:266–273.

19. Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988; 239:487–491.

20. Bishop RF, Cipriani E, Lund JS, Barnes GL, Hosking CS. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984; 19:447–452.

21. Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006; 85:74–78.

22. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007; 449:819–826.

23. Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000; 71:1528–1534.

24. Delanghe JR, Langlois MR, De Bacquer D, Mak R, Capel P, Van Renterghem L, et al. Discriminative value of serum amyloid A and other acute-phase proteins for coronary heart disease. Atherosclerosis. 2002; 160:471–476.

25. Graziani F, Cei S, La Ferla F, Vano M, Gabriele M, Tonetti M. Effects of non-surgical periodontal therapy on the glomerular filtration rate of the kidney: an exploratory trial. J Clin Periodontol. 2010; 37:638–643.

26. Vuletic S, Taylor BA, Tofler GH, Chait A, Marcovina SM, Schenck K, et al. SAA and PLTP activity in plasma of periodontal patients before and after full-mouth tooth extraction. Oral Dis. 2008; 14:514–519.

27. Clifton PM, Mackinnon AM, Barter PJ. Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J Lipid Res. 1985; 26:1389–1398.

28. O'Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, et al. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005; 25:785–790.
29. Barassi A, Pezzilli R, Morselli-Labate AM, Lombardi F, Belletti S, Dogliotti G, et al. Serum amyloid a and C-reactive protein independently predict the recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. Can J Cardiol. 2012; 28:537–541.

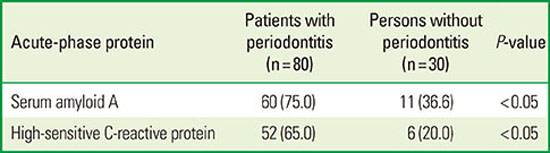
30. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001; 72:1221–1227.

31. Linden GJ, McClean K, Young I, Evans A, Kee F. Persistently raised C-reactive protein levels are associated with advanced periodontal disease. J Clin Periodontol. 2008; 35:741–747.

32. Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000; 79:49–57.

33. Unlü Y, Karapolat S, Karaca Y, Kiziltunc A. Comparison of levels of inflammatory markers and hemostatic factors in the patients with and without peripheral arterial disease. Thromb Res. 2006; 117:357–364.

34. Kosuge M, Ebina T, Ishikawa T, Hibi K, Tsukahara K, Okuda J, et al. Serum amyloid A is a better predictor of clinical outcomes than C-reactive protein in non-ST-segment elevation acute coronary syndromes. Circ J. 2007; 71:186–190.

35. Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005; 76:11 Suppl. 2089–2100.

36. Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003; 74:1007–1016.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download