CURRENT SURGE IN THE STEM CELL INDUSTRY IN KOREA
Stem cell therapy is considered as the most promising field of future medicine by virtue of its possibility to treat debilitating and degenerative diseases, which are currently incurable with conventional therapies. In addition to its importance in medicine, stem cell therapy holds great promises in potential applications that have a high industrial value. Currently, the annual world market has already reached 5 billion US$, and their market is rapidly growing. Moreover, many countries around the world are highly interested in technological and industrial initiatives in stem cell therapeutics. Recently, Korean government has launched into political movements towards active investments in the stem cell industry. The Korean president described the stem cell field as a "new growth engine" for the nation's economy. Moreover, legislative movements are being proposed for simplifying the authorization process of cell therapy products in order to lower the barriers against industrialization. Overall, governmental efforts to boost the nation's industrial competitiveness by increasing investments in stem cell research have been well received. However, there are also significant controversies regarding the simplification of the authorization process for cell therapeutics.
SIGNIFICANCE OF CLINICAL TRIALS IN THE AUTHORIZATION OF CELL THERAPY PRODUCTS
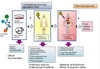
Generally, the companies that develop cell therapeutics are requested to file the documents regarding the safety and efficacy of their products in order to obtain approval for market sales. Then, the validity of the cell product as a drug is examined by a government inspection agency (currently, the Food and Drug Administration); to determine if the proposed drug is sufficiently safe for patient treatments, or if it is capable of providing an objective therapeutic benefit. These processes are performed through phase I to phase lll clinical trials. The phase I and phase ll clinical trials chiefly focus on safety issues such as potential contamination and/or infection, immediate immune responses, optimal dosage, or unexpected side effects in the human body that had not been observed in the animal studies. Therefore, clinical trials up to these stages are fairly preliminary, and assessment on the definite therapeutic effects as shown by statistical analyses is not included at this stage of trials; this assessment is covered in the phase lll clinical trials. Thus, phase lll trials include statistical verification on the concrete therapeutic benefits in patients administered with the candidate drug in direct comparison to the patients' subjective placebo effects. In addition, phase lll trials focus on the long-term safety in large groups of patients exposed to the candidate drug. Hence, the phase lll clinical trials serve as a scientific basis for governmental insurance on the safety and efficacy of the candidate drug before it is approved to be marketed to consumers (Fig. 1).
EFFICIENCY OR EFFICACY: WHAT MATTERS MORE?
Considering the significance of phase lll clinical trials for public interests, it is important to note that to some extent, discrepancies do exist between the general public consensus and the current political trends towards simplification of the authorization process for cell therapeutics. For example, one legislative proposal pending in the National Assembly of Korea proposes that stem cell therapeutics targeting rare diseases should be approved solely based on the phase I clinical trials and investigative clinical trials; another proposal suggests that stem cell therapeutics from autologous donors should be exempted from phase lll clinical trials. Thus, these proposals advocate exemption of phase lll clinical trials to increase the efficiency of industrializing stem cell therapeutics. However, certain cautionary measures need to be taken considering the potential situations which could arise from such regulatory systems. Under such regulatory systems, governmental agencies would be forced to stamp the approval documents without having sufficient opportunity to verify the therapeutic effects (efficacy) or long-term toxicities of a drug; however, the public would take it as authorization based on the safety and therapeutic effects. If the efficacies are sub-optimal, patients would be further victimized, by not having medical benefit but having spent money on insufficiently validated cell therapy drugs. It should be noted that the Japanese governmental agency was once considered to be responsible for damages to patients that were partly caused by inadequate authorization of drug entities. Insufficient inspection of new cell therapy drugs for improving efficiencies of industrial processes can pose great risks to the nation.
Interestingly, due to expectations from political parties, the industrial sectors in Korea that deal with cell therapeutics are generally opposed to such legislative proposals. The companies are concerned about the possible decline of the credibility of their cell therapy products in international and domestic markets under such conditions, which will hamper their overall market competitiveness. Notably, the Korean FDA has approved 3 stem cell therapy products using the standard regulatory system, all of which are first runners in the global market (Table 1). Thus, further industrial competitiveness for cell therapeutics in Korea should be sought from technological advances and proven therapeutic efficacy of the products rather than from simplification of regulatory systems. Further, loosening of regulatory system is likely to impede industrial benefits as well as public health security rather than augmenting the efficiency of industrialization of cell therapy products of Korean companies. These findings show that "efficacy" is much more important than "efficiency" with regard to stem cell therapeutics.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download