The characterization of natural killer (NK) cells initially occurred in the 1970s and 1980s as a result of efforts by investigators such as Rolf Kiessling and Ronald Heberman. It was observed that a certain population of cells, freshly isolated from normal, unimmunized hosts, could lyse allogeneic tumor cells without sensitization. At the time, these cells were just considered to be a population of non-T, non-B lymphocytes. After the introduction of modern technologies such as monoclonal antibody (mAb) production technology and flow cytometry, the population has been identified as NK cells, phenotypically defined as CD56+ CD3-. Nowadays, it is well known that NK cells have a role as killer cells as well as immunoregulatory cells secreting cytokines and chemokines. Contrary to traditional views, NK cells have recently been shown to require a process called "licensing" or "education" to acquire full function by recognition of self-MHC class I during maturation.
NK cells are derived from CD34+ hematopoietic progenitor cells and have the morphology of large granular lymphocytes. They can directly kill target cells via the perforin-granzyme pathway, antibody-dependent cellular cytotoxicity (ADCC), and death receptor ligand-induced apoptosis. Such ligands include tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas ligand. Human NK cells can be classified into two subsets; CD56dim (90%) and CD56bright (10%). The CD56dim subset has high cytotoxic activity and expresses a low-affinity receptor for the constant region of immunoglobulin G, FcγRIIIa (CD16), while the CD56bright subset has the capacity to produce abundant cytokines.
It is now well known that NK cell cytotoxicity is tightly regulated by a balance between activating and inhibitory signals. However, initially NK cells were considered to be effector cells with the ability to conduct nonspecific killing of tumor target cells. In 1986, Klas Karre and colleagues first proposed their "missing self" hypothesis. According to this theory, inhibitory receptors (killer cell immunoglobulin-like receptor, KIRs) of NK cells specifically recognize self-MHC class I molecules expressed by normal cells, and this recognition leads to the inhibition of their killing activity [1]. If the self-MHC class I molecules on normal cells are altered or absent because of viral infection or transformation, the inhibitory signal will decrease, leading to cytotoxic activity against the target cell. Recent studies have identified NK cell inhibitory receptors that recognize non-MHC-molecule ligands, although the best described inhibitory receptors recognize MHC class I molecules [1].
For many years NK cells were considered to be controlled only via inhibitory mechanisms. However, these mechanisms could not fully explain the fact that some MHC-negative cells are resistant to an NK cell attack while certain NK-susceptible targets express a normal MHC class I repertoire. This led to the conclusion that NK cell activation requires more than just the absence of inhibitory signals and that, ideally, the target must also express specific ligands for NK activating receptors [1]. A good example is the NKG2D activating receptor that recognizes stress-induced self-proteins such as MICA/B and ULBP 1-5, which are upregulated in infected or transformed cells. This is known as "induced self". It is now well established that NK cells require specific activating signals as well as the absence of inhibitory signals in order to exhibit killing activity.
In 1982, Rosenberg and colleagues demonstrated that autologous lymphokine-activated killer (LAK) cells, prepared by culturing peripheral blood lymphocytes (PBL) in interleukin-2 (IL-2) for 2-3 days, could lyse freshly isolated autologous solid tumor cells in vitro. This killing effect was predominantly mediated by NK cells [2, 3]. Based on the experimental and clinical studies by this group, several clinical trials on LAK cells, mostly in combination with IL-2, were performed by the end of the 1990s. Although a clinical response rate of about 15-20% was reported, LAK therapy in this form was discarded due to its significant toxicity and non-superiority to IL-2 therapy alone. The failure of these autologous LAK and NK cell-based therapies are explained by the presence of inhibitory KIRs and their role in preventing NK cell killing of "self"-MHC-expressing tumor cells or IL-2-induced expansion of regulatory T cells that directly inhibit the NK cell killing effect [2].
This failure led to the investigation of the use of allogeneic NK cells in cellular therapy. In 2002, It was reported that KIR-ligand mismatch between patients and their donors was associated with improved outcomes in myeloid leukemia after T cell depleted haploidentical hematopoietic stem cell transplantation (HCT). They used stem cells or NK cells from donors that were mismatched for their KIR ligand expression pattern as compared to the MHC profile of the host recipient. This created a situation where the newly arrived donor NK cells were released from their KIR inhibition against tumor cells that lacked the appropriate MHC molecules. Additional clinical trials support the association between KIR-ligand mismatch and survival advantage after unrelated donor HCT when anti-thymocyte globulin is used as part of graft vs. host disease prophylaxis [3].
In addition to the use of allogeneic NK cells in the context of HCT, adoptive transfer of allogeneic NK cells from haploidentical donor has also been used. In 2005, Miller and colleagues showed that haploidentical donor-related NK cell infusions expanded in vivo were safe and effective, especially in patients with refractory acute myeloid leukemia with KIR-ligand mismatched status who received a lymphocyte-depleting regimen of cyclophosphamide and fludarabine (Hi-Cy/Flu). However, contrary to their initial report, in 2008, this group reported that the clinical efficacy of the treatment did not correlate with KIR-ligand mismatched status [3]. Adoptive transfer protocols are also now using NK cell donor lymphocyte infusions after haploidentical HCT to consolidate engraftment or adoptively transferred NK cells to augment HCT [3]. Recently ex vivo expansion techniques for NK cells have undergone marked improvement. Thus, improved clinical outcomes might be anticipated if ex vivo expanded NK cells were employed in adoptive transfer protocols.
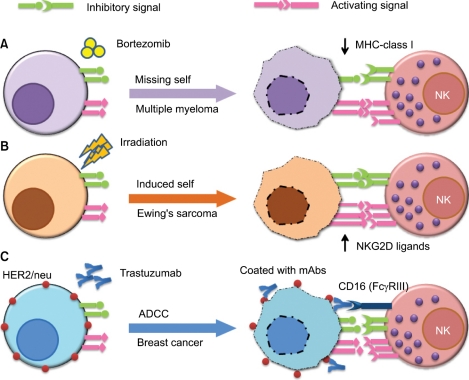
Recently there is some evidence of potential synergistic effects of combinational cancer therapies against hematologic malignancies and solid tumors (Fig. 1). One approach would be to combine NK cell-based immunotherapy with chemotherapy. It was shown that the bortezomib, a proteasome inhibitor that is clinically approved for the treatment of refractory/relapsed myeloma, down-regulates the cellsurface expression of HLA class I on target cells and enhances natural killer cell-mediated lysis of myeloma tumor targets. Moreover, clinically relevant bortezomib concentrations did not affect NK-cell function. According to their findings, this combination therapy has important therapeutic implications for multiple myeloma and other NK cell-sensitive malignancies in the context of adoptively - transferred allogeneic and autologous NK cells.
Another example is combining NK cell therapy with radiation therapy because irradiation-induced tissue injury is known to increase the expression of NK activating ligands (e.g., NKG2D ligands) on malignant cells, thus rendering tumors more susceptible to NK cell cytotoxic activity. Cho et al. [4] recently showed that Ewing's sarcoma cells are highly sensitive to expanded allogeneic NK cells. Radiation therapy has been shown to significantly enhance the NK cell-mediated killing of these tumors in an orthotopic murine model.
Another approach is to combine NK cell-based therapy with mAb therapy because NK cells express an activating Fc receptor that recognizes the constant region of tumor-bound antibodies. This allows NK cells to kill mAb-coated target cells through ADCC. Clinical examples of this effect include patients with CD20+ lymphoma who have been treated with rituximab (Rituxan™) or patients with HER2/neu expressing breast cancers who have been treated with trastuzumab (Herceptin™). Dr. William Carson's group has shown that co-administration of immunomodulatory cytokines (e.g., IL-12) can enhance the effects of anti-tumor mAbs via the activation of NK cells in vitro and in the context of a phase I trial of IL-12 and trastuzumab [5] and a follow-up trial of IL-12 in combination with trastuzumab plus paclitaxel for patients with HER2 overexpressing cancers.
In conclusion, NK cell-based immunotherapy is a promising therapy for solid and hematologic cancers that can potentially be combined with chemotherapy, radiation, or mAb therapy.
References
1. Stern-Ginossar N, Mandelboim O. Lotze MT, Thomson AW, editors. Receptors on NK cells. Natural killer cells: basic science and clinical application. 2010. 1st ed. London, UK: Academic Press;p. 155–168.

2. Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008; 9:486–494. PMID: 18425105.

3. Cooley S, Miller JS. Lotze MT, Thomson AW, editors. Clinical trials of NK cells for cancer. Natural killer cells: basic science and clinical application. 2010. 1st ed. London, UK: Academic Press;p. 555–570.

4. Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010; 16:3901–3909. PMID: 20542985.

5. Bekaii-Saab TS, Roda JM, Guenterberg KD, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Cancer Ther. 2009; 8:2983–2991. PMID: 19887543.

Fig. 1
Representative examples of combinatorial approaches of natural killer (NK) cell immunotherapy with chemotherapy, radiation, or monoclonal antibody (mAb) therapy. One approach (A) would be to combine NK cell immunotherapy with chemotherapy (e.g., bortezomib), which downregulates cell surface expression of human leukocyte antigen (HLA) class I on target cells (missing self) and enhances NK cell-mediated lysis of tumor targets. Another approach (B) is to combine NK cell therapy with radiation therapy, which is known to increase the expression of NK activating ligands (e.g., NKG2D ligands MICA and MICB) on malignant cells (induced self), thus rendering tumors more susceptible to NK cell cytotoxic activity. The third approach (C) is to combine NK cell immunotherapy with mAb therapy (e.g., the anti-HER2/neu mAb trastuzumab). NK cells express an activating Fc receptor (FcγRIIIa, CD16) that recognizes the constant region of IgG and allows them to kill antibody-coated target cells via antibody-dependentcell-mediated cytotoxicity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download