Abstract
Background:
Cyclosporine (CSA) plus 4 doses of methotrexate (MTX) is the commonly used regimen for GVHD prophylaxis. It has been previously found that the omission of the day +11 dose of MTX was associated with an increased risk of acute GVHD in the allogeneic BMT setting. However, little is known about its impact in the PBSCT setting.
Methods:
Of the 68 patients, 30 patients (44%) received 4 doses of MTX (the MTX4 group), while 38 patients (56%) received less than 4 doses (the MTX3 group) because of their severe mucositis, hepatic dysfunction or renal failure.
Results:
The cumulative incidence of acute GVHD was 60% in the MTX4 and 86% in the MTX3 group (P=0.038), while that of grade III and IV acute GVHD was 7% in the MTX4 group and 39% in the MTX3 group (P=0.017). Of the 61 patients evaluated for chronic GVHD, the cumulative incidence of chronic GVHD was 54% in the MTX4 group and 97% in the MTX3 group (P=0.001), while that of extensive chronic GVHD was 26% in the MTX4 group and 63% in the MTX3 group (P=0.004). There were no differences in the overall survival and the incidence of relapse between the two groups. On multivariate analyses, MTX3 was a poor prognostic factor in terms of acute GVHD and extensive chronic GVHD.
Conclusion:
This study suggested that omitting day +11 MTX and the clinical situation of the MTX3 group seemed to be associated with an increased incidence of acute and chronic GVHD. Accordingly, administration of day +11 MTX accompanied by active treatment of mucositis may prevent GVHD in the allogeneic PBSCT setting, but we need to conduct a large scale prospective study.
Go to : 
REFERENCES
1). Russell NH., Hunter A., Rogers S., Hanley J., Anderson D. Peripheral blood stem cells as an alternative to marrow for allogeneic transplantation. Lancet. 1993. 341:1482.

2). Sohn SK., Jung JT., Kim DH, et al. Prophylactic growth factor-primed donor lymphocyte infusion using cells reserved at the time of transplantation after allogeneic peripheral blood stem cell transplantation in patients with high-risk hematologic malignancies. Cancer. 2002. 94:18–24.

3). Kim JG., Sohn SK., Kim DH, et al. Impact of ABO incompatibility on outcome after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2005. 35:489–95.

4). Champlin RE., Schmitz N., Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2000. 95:3702–9.
5). Bensinger WI., Martin PJ., Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001. 344:175–81.

6). Mielcarek M., Martin PJ., Torok-Storb B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 1997. 89:1629–34.

7). Tanimoto TE., Yamaguchi T., Tanaka Y, et al. Comparative analysis of clinical outcomes after allogeneic bone marrow transplantation versus peripheral blood stem cell transplantation from a related donor in Japanese patients. Br J Haematol. 2004. 125:480–93.

8). Levine JE., Wiley J., Kletzel M, et al. Cytokine-mobilized allogeneic peripheral blood stem cell transplants in children result in rapid engraftment and a high incidence of chronic GVHD. Bone Marrow Transplant. 2000. 25:13–8.

9). Wang M., Han M., Feng S. Comparison of clinical outcome between allogeneic peripheral blood stem cell transplantation and bone marrow transplantation. Zhonghua Xue Ye Xue Za Zhi. 1999. 20:427–30.
10). Nagatoshi Y., Kawano Y., Watanabe T, et al. Hematopoietic and immune recovery after allogeneic peripheral blood stem cell transplantation and bone marrow transplantation in a pediatric population. Pediatr Transplant. 2002. 6:319–26.

11). Kumar S., Wolf RC., Chen MG, et al. Omission of day +11 methotrexate after allogeneic bone marrow transplantation is associated with increased risk of severe acute graft-versus-host disease. Bone Marrow Transplant. 2002. 30:161–5.

12). Storb R., Deeg HJ., Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986. 314:729–35.

13). Aschan J., Ringden O., Sundberg B., Gahrton G., Ljungman P., Winiarski J. Methotrexate combined with cyclosporin A decreases graft-versus-host disease, but increases leukemic relapse compared to monotherapy. Bone Marrow Transplant. 1991. 7:113–9.
14). Ringden O., Horowitz MM., Sondel P, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993. 81:1094–101.

15). Storb R., Deeg HJ., Pepe M, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989. 73:1729–34.

16). Sohn SK., Kim JG., Sung WJ, et al. Harvesting peripheral blood stem cells from healthy donors on 4th day of cytokine mobilization. J Clin Apher. 2003. 18:186–9.

17). Sohn SK., Kim JG., Chae YS, et al. Large-volume leukapheresis using femoral venous access for harvesting peripheral blood stem cells with the Fenwal CS 3000 Plus from normal healthy donors: predictors of CD34+ cell yield and collection efficiency. J Clin Apher. 2003. 18:10–5.

18). Sohn SK., Kim JG., Seo KW, et al. GM-CSF-based mobilization effect in normal healthy donors for allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2002. 30:81–6.

19). Przepiorka D., Weisdorf D., Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995. 15:825–58.
20). Shulman HM., Sullivan KM., Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980. 69:204–17.
21). Deeg HJ., Spitzer TR., Cottler-Fox M., Cahill R., Pickle LW. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991. 7:193–8.
22). Atkinson K., Downs K. Omission of day 11 methotrexate does not appear to influence the incidence of moderate to severe acute graft-versus-host disease, chronic graft-versus-host disease, relapse rate or survival after HLA-identical sibling bone marrow transplantation. Bone Marrow Transplant. 1995. 16:755–8.
23). Nash RA., Pepe MS., Storb R, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992. 80:1838–45.
Go to : 
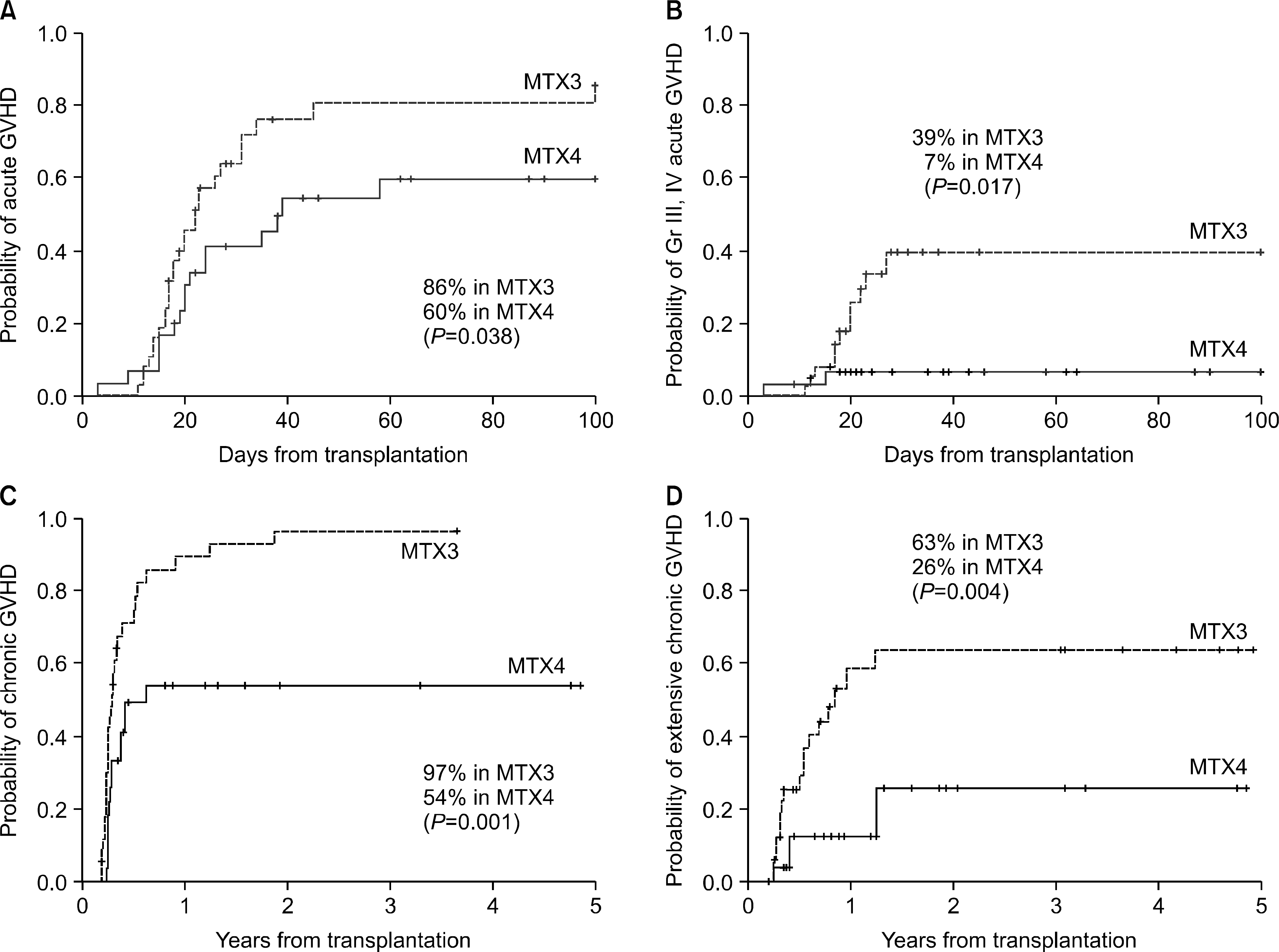 | Fig. 1The cumulative incidence of acute and chronic GVHD after HLA-identical allogeneic PBSCT (n=68). The group with 4 doses of methotrexate showed significantly lower incidence of acute GVHD (A), grade III-IV acute GVHD (B), overall chronic GVHD (C), and extensive chronic GVHD (D). |
Table 1.
Patients’ characteristics and transplantation outcomes
Table 2.
Patients’ characteristics and transplantation outcomes according to the MTX dose
| MTX3, n (%) | MTX4, n (%) | P value | ||
|---|---|---|---|---|
| No. of patients | 38 (59) | 30 (41) | ||
| Sex | Male/female | 20/18 | 22/8 | 0.041 |
| Age | Median (range) | 36 years (17~54) | 37 years (16~58) | 0.828 |
| Diagnosis | Acute myelogenous leukemia | 24 (63.2) | 11 (36.7) | 0.030 |
| Acute lymphoblastic leukemia | 3 (7.9) | 3 (10.0) | 0.761 | |
| Aplastic anemia | 1 (2.6) | 9 (30.0) | 0.002 | |
| Chronic myelogenous leukemia | 2 (5.3) | 3 (10.0) | 0.457 | |
| Myelodysplastic syndrome | 2 (5.3) | 1 (3.3) | 0.700 | |
| Non-Hodgkin's lymphoma | 4 (10.5) | 2 (6.7) | 0.577 | |
| Multiple myeloma | 1 (2.6) | 0 (0) | 0.371 | |
| Others | 1∗ (2.6) | 1† (3.3) | - | |
| Disease risk | High risk | 20 (52.6) | 12 (40.0) | 0.300 |
| Conditioning | Myeloablative | 31 (81.6) | 19 (63.3) | 0.090 |
| Nonmyeloablative | 7 (18.4) | 11 (36.7) | ||
| LV rescue | Done | 7 (18.4) | 18 (60.0) | 0.001 |
| Not done | 31 (81.6) | 12 (40.0) | ||
| Stem cell source | Peripheral blood | 38 (100) | 30 (100) | 1.00 |
| Cell dose | MNC (×108/kg) | 8.65±0.52 | 10.13±0.86 | 0.131 |
| CD34+ (×106/kg) | 9.14±0.81 | 8.48±0.98 | 0.603 | |
| Engraftment | WBC, median (range) | 14 days (10~29) | 13.5 days (10~25) | 0.344 |
| Platelet, median (range) | 14 days (9~56) | 12.5 days (10~34) | 0.263 | |
| CMV reactivation | Yes | 15 (39.5) | 15 (50.0) | 0.385 |
| GVHD | Acute GVHD | 28 (85.6) | 16 (60.0) | 0.038 |
| Acute GVHD Gr III, IV | 11 (39.4) | 2 (6.8) | 0.017 | |
| Chronic GVHD | 30 (96.7), n=34 | 14 (54.2), n=27 | 0.001 | |
| Chronic GVHD, extensive | 17 (63.4), n=34 | 5 (25.6), n=27 | 0.004 | |
| Survival | Overall survival | 16 (42.1±0.49) | 18 (60.0±0.50) | 0.143 |
| Relapse | 12 (31.6) | 10 (33.3) | 0.878 | |
| Non-relapse mortality | 14 (36.8) | 6 (20.0) | 0.130 | |
| Cause of death | Disease progression | 8 (21.0) | 5 (16.7) | 0.648 |
| Infection | 9 (23.7) | 5 (16.7) | 0.477 | |
| GVHD | 4 (10.5) | 0 (0) | 0.067 | |
| Others | 1 (2.6) | 1 (3.3) | - |
Table 3.
Acute and chronic GVHD incidence according to leucovorin rescue
Table 4.
Multivariate survival analyses in terms of acute and chronic GVHD




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download