Dear Editor:
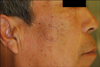
A 62-year-old man presented with a 5-month history of solitary subcutaneous nodule on the right cheek that. On physical examination, there was a 2-cm flesh-toned subcutaneous round nodule on the right cheek (Fig. 1). The mass was soft and somewhat fluctuant on palpation without tenderness. Histopathologic examination revealed multiple well-circumscribed tumor nests with solid, lobular, and microcystic structures (Fig. 2A). A large portion of tumor cells displayed serous acinar differentiation with cytoplasmic granules, termed cytoplasmic zymogen-like granules (Fig. 2B). The cytoplasmic granules were periodic acid-Schiff (PAS)-positive and diastase-resistant (Fig. 2C). Moderate nuclear pleomorphism and hyperchromasia were present. Immunohistochemical staining revealed that the tumor cells were immunopositive for cytokeratin, α-1 antitrypsin, and α-1 antichymotrypsin (Fig. 2D~F), while they were immunonegative for S100 protein. We diagnosed the patient with secondary cutaneous acinic cell carcinoma (ACC) that had locally advanced from the right parotid gland.
ACC is a malignant salivary gland neoplasm that accounts for 20% of all salivary gland malignancies, and 90% of cases arise in the parotid gland. The onset incidence peaks in the fifth and sixth decades although age at onset varies from 5 years to over 80 years of age1. ACC presents as a painless subcutaneous nodule. In rare cases, pain and tenderness can occur intermittently. Facial paralysis, which occurs in 11% of cases, is usually a serious prognostic sign2. Cutaneous ACC metastasis is rare, with only a few reported cases3.
Microscopically, primary tumors can present with four characteristic growth patterns: solid/lobular, microcystic, papillary cystic, and follicular, with several patterns often seen in the same tumor. The diagnostic feature of ACC is the presence of at least some neoplastic cells demonstrating serous acinar differentiation, which is characterized by cytoplasmic zymogen secretory granules4. These tumor cells are polygonal with lightly basophilic to amphophilic fine granules in the cytoplasm. The granules are characteristically PAS-positive and diastase-resistant. In addition to serous cells, there may also be clear cells, intercalated duct cells, vacuolated cells, and non-specific glandular cells4. Tumor size, degree of differentiation, and infiltrative growth patterns are useful in predicting clinical courses5. Immunohistochemistry showed that tumor cells can be positive for cytokeratin, α-1 antichymotrypsin, α1-antitrypsin, and lysozyme, with approximately 10% of cells positive for S100 protein. However, there is no specific immunoprofile, and immunohistochemistry has little impact on diagnosis4.
ACC diagnosis is usually confirmed with fine-needle aspiration biopsy, and surgical excision is the treatment of choice. Radiation therapy is generally reserved for more aggressive or recurrent tumors, while neck dissection is performed in patients with clinically positive nodes. In one study, the 5-year survival rates were 83.3% (observed) and 91.4% (disease-specific), and poor survival was associated with high grade, patient age >30 years, and metastatic disease1.
We have here reported a rare case of secondary cutaneous ACC presenting as solitary subcutaneous nodule on the right cheek in a 62-year-old man. When examining dermal or subcutaneous tumors of the face, tumors originating from the salivary gland should be considered.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download