Abstract
Background
Thyroid autoimmunity has been increasingly reported to be associated with chronic urticaria (CU), and the possible clinical benefit of thyroid hormone or anti-thyroid drugs in some CU patients with autoimmune thyroid disease has been studied. However, the effect of thyroid hormone or anti-thyroid drugs on clinical symptoms of CU remains unclear.
Objective
We investigated the clinical response of urticaria to the treatment of thyroid dysfunction.
Methods
Medical records of patients with CU evaluated for thyroid autoimmunity and thyroid function were collected and reviewed.
Results
Of 184 patients with CU, 43 patients (23.4%) had thyroid autoantibodies and 26 patients (14.1%) had thyroid dysfunction. While none of the five patients with Graves' disease showed any improvement of urticaria after treatment with anti-thyroid drugs, two of the 10 patients with Hashimoto's thyroiditis showed improved urticaria after being treated with levothyroxine.
Chronic urticaria (CU) is defined as recurrent episodes of wheals for at least 6 weeks. CU is a common disorder with an estimated prevalence of 0.5% to 5% in the general population1. Although there are several known causes of CU, including physical stimuli, allergy, systemic illness, drug or infection, no definitive cause of CU can be identified despite a thorough medical evaluation in 80%~90% of cases2.
Since the 1980s, an increasing number of reports have suggested an association of CU with autoimmune disease. The presence of a serum factor that induced an immediate wheal-and-flare response following the intradermal injection of autologous serum from 44%~58% of patients with CU has been reported and the presence of autoantibodies capable of inducing mast cell degranulation was noted34. In the 1990s, studies demonstrated the presence of immunoglobulin G (IgG) autoantibodies directed against the α subunit of the high-affinity IgE receptor5 and autoantibodies directed against IgE6 in sera from 45%~55% of patients with CU. These autoantibodies have been shown to activate blood basophils and cutaneous mast cells, resulting in the release of histamine and other proinflammatory mediators7.
The association between CU and various autoimmune disorders, including thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, Sjogren's syndrome, and type 1 diabetes has been recognized as indirect evidence of an autoimmune entity of CU8. Among them, autoimmune thyroid diseases were the most commonly reported autoimmune diseases in patients with CU9. Thyroid autoantibodies, indicators for the diagnosis of autoimmune thyroid disease, were found in 12%~37% of patients with CU1011. The role of thyroid autoantibodies in the pathogenesis of CU is debatable12. Several studies have suggested that epitopic cross-reactivity between thyroid autoantibodies and other autoantibodies found in CU lack evidence and thyroid autoantibodies themselves have no in vivo effect on mast cell and basophil releasability1314.
After a high prevalence of thyroid autoantibodies in patients with CU was reported, resolution of urticarial after thyroid hormone replacement therapy in some patients with concomitant thyroid autoimmunity was reported15, and some authors have subsequently recommended the therapeutic use of thyroid hormone in patients with CU16. However, to our knowledge, no studies have investigated the clinical response of urticaria to the treatment of coexisting thyroid diseases in a Korean population. In this study, we investigated the clinical response of urticaria to the treatment of thyroid dysfunction in Korean patients with CU.
We retrospectively reviewed the records of patients with CU seen at the Department of Dermatology or Allergy & Clinical Immunology at Dongguk University Ilsan Hospital (Goyang, Korea) from July 2005 to June 2013. The diagnosis of CU was established by the presence of intermittent or continuous wheals for at least 6 weeks. Patients were excluded if they had primarily physical urticaria, food or drug-related urticaria, or urticaria vasculitis.
Demographic data and laboratory data were collected. Serum-free T4 (FT4) (normal, 0.93~1.70 ng/dl), thyroid-stimulating hormone (TSH) (normal, 0.27~4.20 µU/ml), anti-thyroperoxidase (TPO) (normal, 0~34.00 IU/dl), and anti-thyroglobulin (TG) (normal, 0~115.00 IU/dl) antibody levels were measured by an electrochemiluminescence immunoassay. Hypothyroidism was diagnosed by laboratory criteria of FT4 <0.9 ng/dl and TSH >4.2 µU/ml. Patients were classified as having subclinical hypothyroidism if they had a normal FT4 level, but had an elevated TSH level17. Hyperthyroidism was diagnosed by laboratory criteria of FT4 >1.70 ng/dl and TSH <0.27 µU/ml. All patients with thyroid dysfunction were examined by an endocrinologist. The clinical course of urticaria was reviewed in accordance with thyroid function. The clinical effectiveness of levothyroxine (or anti-thyroid drugs) in CU patients was defined as elimination of daily spontaneous wheals or a significant reduction of medication to control daily spontaneous wheals14. The clinical response of urticaria was evaluated until the normalization of thyroid function after treatment with levothyroxine (or anti-thyroid drugs).
Data was analyzed using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA). Statistical significance was assessed using the chi-square test and p-values<0.05 were considered statistically significant.
In total, 184 patients with CU were enrolled in the study, with a mean age of 37.8 years (range, 12~72 years). Of the 184 patients, 56 were men (mean age, 37.8 years; range, 12~72 years) and 128 were women (mean age, 37.8 years; range, 12~68 years). All patients reported symptoms of CU for a mean duration of 14.6 months (range, 2 months to 30 years).
Thyroid autoantibodies (anti-TPO and/or anti-TG) were detected in 43 patients (23.4%) (5 men, 38 women). Of the 43 patients, 25 had increased levels of anti-TPO antibody, 38 had increased levels of anti-TG antibody, and 20 had increased levels of both anti-TPO and anti-TG antibodies (Table 1). The frequency of thyroid autoantibodies in women (29.7%, 38/128) was significantly higher than that in men (8.9%, 5/56) (p=0.002) (Table 1). In patients with thyroid autoantibodies, immunologic and other laboratory testing was not significant (Table 2). Rheumatoid factor, as well as complement C3 and C4, were normal in all patients. Erythrocyte sedimentation rate was elevated in three patients. Three patients had a persistent titer of anti-nuclear antibody ≥1:100 with no other evidence of connective tissue disorder.
Of the total 184 patients with CU, 26 patients (14.1%) were found to have thyroid dysfunction (3 men, 23 women) and the remaining 158 patients (85.9%) were euthyroid. Of the 26 patients with thyroid dysfunction, eight were hyperthyroidic, 10 were hypothyroidic, and eight were classified as having subclinical hypothyroidism (Table 3). The frequency of thyroid dysfunction in women (18.0%, 23/128) was significantly higher than that in men (5.4%, 3/56) (p=0.024) (Table 3). The frequency of thyroid dysfunction in patients with thyroid autoantibodies (48.8%, 21/43) was significantly higher than that in patients without thyroid autoantibodies (3.6%, 5/141) (p<0.001) (Table 4).
Of the eight patients with hyperthyroidism, five were diagnosed with Graves' disease and treated with methimazole (3 patients) or propylthiouracil (2 patients). Although thyroid hormone levels were normalized after treatment with anti-thyroid drugs, none of the five patients showed any improvement of their respective urticaria. All 10 patients with hypothyroidism were diagnosed with Hashimoto's thyroiditis and treated with levothyroxine. As TSH levels decreased after treatment with levothyroxine, two of the 10 patients with Hashimoto's thyroiditis showed improvement in their urticaria (Table 5). All eight patients with subclinical hypothyroidism were not treated with levothyroxine, but were given repeated thyroid function tests due to the probability of developing a thyroid dysfunction. None of the eight patients with subclinical hypothyroidism experienced remission of urticaria during the follow-up period.
Thyroid autoimmunity is characterized by the formation of thyroid autoantibodies. Thyroid autoantibodies, indicators for the diagnosis of autoimmune thyroid disease, were found in 3%~9% of the general population, with the prevalence increasing markedly in women above the age of 45 years18. The prevalence of thyroid autoantibodies in the general Korean population was reported to be 8.9%19. In this study, we detected thyroid autoantibodies in 23.4% of patients with CU. This result is consistent with previous reports in which thyroid autoantibodies were found in 12%~37% of CU patients (Table 6)10111617202122232425. Our study showed that the prevalence of thyroid autoantibodies was significantly (p<0.05) higher in Korean patients with CU than the assumed prevalence (less than 9%) in the general Korean population.
The clinical presentation of thyroid autoimmunity varies from hypothyroidism in Hashimoto's thyroiditis to hyperthyroidism in Graves' disease. Hypothyroidism or subclinical hypothyroidism is more frequent than hyperthyroidism in patients with CU26. In this study, the prevalence of hyperthyroidism, hypothyroidism, and subclinical hypothyroidism in patients with CU was 4.3% (men 1.8%, women 5.5%), 5.4% (men 1.8%, women 7.0%), and 4.3% (men 1.8%, women 5.5%), respectively. The prevalence of hyperthyroidism, hypothyroidism, and subclinical hypothyroidism in the general Korean population has been reported to be 0.6% (men 0.4%, women 0.8%), 0.3% (men 0.1%, women 0.5%), and 1.8% (men 1.1%, women 2.7%), respectively27. Although it is difficult to compare the prevalence of thyroid dysfunction reported by different groups due to the differences in the study population, our data showed a high prevalence of thyroid dysfunction in patients with CU. The incidence of thyroid dysfunction has been reported as 12%~29% in CU patients and 24%~55% in thyroid antibody-positive patients with CU (Table 7)17202829. Our results confirmed these previous studies showing that thyroid dysfunction was present in 14.1% of patients with CU and that the prevalence increased to 48.8% if CU was accompanied by thyroid autoantibodies.
In this study, two of the 10 patients with Hashimoto's thyroiditis showed improved urticaria after treatment with levothyroxine, whereas none of five patients with Graves' disease showed any improvement of urticaria after treatment with anti-thyroid drugs. While the remission of urticaria after treatment with anti-thyroid drugs in patients with Graves' disease has been reported in only a few anecdotal cases, the resolution of urticaria after levothyroxine treatment in patients with Hashimoto's thyroiditis has been reported by several authors (Table 8)151630313233. Although treatment of thyroid dysfunction was effective in a minority of patients with CU, the results suggest a causal relationship between CU and thyroid autoimmunity. The underlying mechanisms by which anti-thyroid drugs alleviate urticaria remains unknown. However, levothyroxine therapy has been suggested to improve urticaria in patients with Hashimoto's thyroiditis14. There is evidence for a relationship between the degree of inflammation in the thyroid and the presence of urticaria34. Rumbyrt et al.14 proposed that an inflammatory response in the thyroid gland leads to a generalized inflammatory state and lowers the threshold of mast cells to other stimuli and Aversano et al.32 hypothesized that TSH might drive the production of proinflammatory cytokines and can regulate the immune response. According to these hypotheses, levothyroxine therapy would reduce the inflammatory response by TSH suppression and thus reduce the symptoms of urticaria35. These hypotheses remain unproven.
While the present study has limitations owing to its retrospective design with a potential of selection bias, it provides important information about the prevalence of thyroid autoantibodies and thyroid dysfunction in Korean CU patients. Although treatment of thyroid dysfunction was effective only in a minority of patients with CU (two of the 10 patients with Hashimoto's thyroiditis), the association with thyroid autoimmunity must be considered in patients with CU. Further studies are needed to determine the effect of thyroid hormones on CU.
Figures and Tables
Table 1
Prevalence of thyroid autoantibodies in patients with chronic urticaria

Table 2
Abnormal results of other laboratory tests in patients with chronic urticaria with thyroid autoantibodies

| Test | Abnormal range | Tested patient (n) | Abnormal patient (n) |
|---|---|---|---|
| Rheumatoid factor | >20 IU/ml | 34 | 0 |
| C3 | >180 mg/dl | 32 | 0 |
| C4 | >40 mg/dl | 40 | 0 |
| Anti-nuclear antibody | ≥1:100 | 38 | 3 |
| ESR | >20 mm/hr | 40 | 3 |
Table 3
Thyroid function in patients with chronic urticaria

Table 4
Thyroid function based on the presence or absence of thyroid autoantibodies in patients with chronic urticaria
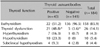
Table 5
Features of the twopatients with Hashimoto's thyroiditis who showed improved urticaria after treatment with levothyroxine

Table 6
Frequency of thyroid autoantibodies in patients with chronic urticaria according to the literature

| Literature | No. of patient | Thyroid autoantibodies | |||
|---|---|---|---|---|---|
| Anti-TPO Ab | Anti-TG Ab | Anti-TPO Ab and anti-TG Ab | Total | ||
| Turktas et al., 1997, Turkey20 | 94 | 11 (11.7) | 9 (9.6) | 9 (9.6) | 11 (11.7) |
| Gaig et al., 2000, Spain16 | 170 | Not specified | 25 (14.7) | ||
| Zauli et al., 2002, Italy21 | 122 | 27 (22.1) | 25 (20.5) | 17 (13.9) | 35 (28.7) |
| Kikuchi et al., 2003, USA22 | 282 | 47 (16.6) | 24 (8.5) | 16 (5.7) | 55 (19.5) |
| Verneuil et al., 2004, France23 | 45 | 8 (17.8) | 8 (17.8) | 4 (8.9) | 12 (26.7) |
| Palma-Carlos and Palma-Carlos, 2005, Portugal11 | 56 | 15 (26.8) | 13 (23.2) | 12 (21.4) | 16 (28.6) |
| Cebeci et al., 2006, Turkey17 | 140 | 27 (19.3) | 23 (16.4) | 9 (6.4) | 41 (29.3) |
| Feibelmann et al., 2007, Brazil10 | 49 | 6 (12.2) | 2 (4.1) | 2 (4.1) | 6 (12.2) |
| Kim et al., 2011, Korea24 | 45 | 11 (24.4) | 6 (13.3) | 4 (8.9) | 13 (28.9) |
| Lee et al., 2013, Korea25 | 194 | 34 (17.5) | 64 (33.0) | 24 (12.4) | 72 (37.1) |
| Present study | 184 | 25 (13.6) | 38 (20.7) | 20 (10.9) | 43 (23.4) |
Table 7
Thyroid function in patients with chronic urticaria with thyroid autoimmunity according to the literature

| Literature | No. of patient | Euthyroidism | Thyroid dysfunction | |||
|---|---|---|---|---|---|---|
| Hyperthyroidism | Hypothyroidism | Subclinical hypothyroidism | Total | |||
| Lenznoff et al., 1983, Canada28 | 17 | 11 (64.7) | 3 (17.6) | 3 (17.6) | - | 6 (35.3) |
| Turktas et al., 1997, Turkey20 | 11 | 5 (45.5) | 1 (9.1) | 3 (27.3) | 2 (18.2) | 6 (54.5) |
| Asero et al., 2003, Italy29 | 66 | 46 (69.7) | 4 (6.1) | 16 (24.2) | 20 (30.3) | |
| Cebeci et al., 2006, Turkey17 | 41 | 31 (75.6) | 2 (4.9) | 1 (2.4) | 7 (17.1) | 10 (24.4) |
| Present study | 43 | 22 (51.2) | 7 (16.3) | 10 (23.3) | 4 (9.3) | 21 (48.8) |
Table 8
Clinical response of hypothyroid patients with chronic urticaria with thyroid autoimmunity to treatment with levothyroxine according to the literature

| Literature | No. of patient | Partial-to-Complete remission | No response |
|---|---|---|---|
| Leznoff and Sussman, 1989, Canada15 | 46 | 8 (17.4) | 38 (82.6) |
| Gaig et al., 2000, Spain16 | 9 | 8 (88.9) | 1 (11.1) |
| Kandeel et al., 2001, USA30 | 14 | 3 (21.4) | 11 (78.6) |
| Levy et al., 2003, Israel31 | 3 | 0 | 3 (100) |
| Aversano et al., 2005, Italy32 | 8 | 8 (100) | 0 |
| Nuzzo et al., 2011, Italy33 | 10 | 0 | 10 (100) |
| Present study | 10 | 2 (20.0) | 8 (80.0) |
References
1. Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969; 81:588–597.
2. Marasoğlu Çelen O, Kutlubay Z, Aydemir EH. Usefulness of the autologous serum test for the diagnosis of chronic idiopathic urticaria. Ann Dermatol. 2014; 26:592–597.

3. Grattan CE, Wallington TB, Warin RP, Kennedy CT, Bradfield JW. A serological mediator in chronic idiopathic urticaria--a clinical, immunological and histological evaluation. Br J Dermatol. 1986; 114:583–590.

4. Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988; 90:213–217.

5. Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993; 328:1599–1604.

6. Grattan CE, Hamon CG, Cowan MA, Leeming RJ. Preliminary identification of a low molecular weight serological mediator in chronic idiopathic urticaria. Br J Dermatol. 1988; 119:179–183.

7. Ferrer M, Kinét JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti-Fc(epsilon) RIalpha (alpha-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998; 101:672–676.

8. Stitt JM, Dreskin SC. Urticaria and autoimmunity: where are we now? Curr Allergy Asthma Rep. 2013; 13:555–562.

9. Fraser K, Robertson L. Chronic urticaria and autoimmunity. Skin Therapy Lett. 2013; 18:5–9.
10. Feibelmann TC, Gonçalves FT, Daud MS, Jorge Ade S, Mantese SA, Jorge PT. Assessment of association between autoimmune thyroid disease and chronic urticaria. Arq Bras Endocrinol Metabol. 2007; 51:1077–1083.
11. Palma-Carlos AG, Palma-Carlos ML. Chronic urticaria and thyroid auto-immunity. Eur Ann Allergy Clin Immunol. 2005; 37:143–146.
13. Mozena JD, Tiñana A, Negri J, Steinke JW, Borish L. Lack of a role for cross-reacting anti-thyroid antibodies in chronic idiopathic urticaria. J Invest Dermatol. 2010; 130:1860–1865.

14. Rumbyrt JS, Katz JL, Schocket AL. Resolution of chronic urticaria in patients with thyroid autoimmunity. J Allergy Clin Immunol. 1995; 96:901–905.

15. Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989; 84:66–71.

16. Gaig P, García-Ortega P, Enrique E, Richart C. Successful treatment of chronic idiopathic urticaria associated with thyroid autoimmunity. J Investig Allergol Clin Immunol. 2000; 10:342–345.
17. Cebeci F, Tanrikut A, Topcu E, Onsun N, Kurtulmus N, Uras AR. Association between chronic urticaria and thyroid autoimmunity. Eur J Dermatol. 2006; 16:402–405.
18. McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012; 42:252–265.

19. Lee MS, Lee DS, Han JS, Cho BY, Koh CS, Lee MH. The prevalence of antithyroid autoantibodies in normal Korean population. Korean J Intern Med. 1985; 28:491–498.
20. Turktas I, Gokcora N, Demirsoy S, Cakir N, Onal E. The association of chronic urticaria and angioedema with autoimmune thyroiditis. Int J Dermatol. 1997; 36:187–190.

21. Zauli D, Grassi A, Ballardini G, Contestabile S, Zucchini S, Bianchi FB. Thyroid autoimmunity in chronic idiopathic urticaria: implications for therapy. Am J Clin Dermatol. 2002; 3:525–528.
22. Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003; 112:218.

23. Verneuil L, Leconte C, Ballet JJ, Coffin C, Laroche D, Izard JP, et al. Association between chronic urticaria and thyroid autoimmunity: a prospective study involving 99 patients. Dermatology. 2004; 208:98–103.

24. Kim JH, Oh TS, Lee SG, Kim IH. Prognostic significance of thyroid autoantibodies in urticaria. Korean J Dermatol. 2011; 49:872–876.
25. Lee SY, Song WJ, Jung JW, Park HW, Cho SH, Min KU, et al. Thyroid autoantibodies and the prognosis of chronic idiopathic urticaria. Allergy Asthma Respir Dis. 2013; 1:151–156.

26. Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012; 129:1307–1313.

27. Chung JH, Kim BJ, Choi YH, Shin MH, Kim SH, Min YK, et al. Prevalence of thyrotoxicosis and hypothyroidism in the subjects for health check-up. J Korean Soc Endocrinol. 1999; 14:301–313.
28. Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983; 119:636–640.

29. Asero R, Lorini M, Tedeschi A. Association of chronic urticaria with thyroid autoimmunity and Raynaud phenomenon with anticentromere antibodies. J Allergy Clin Immunol. 2003; 111:1129–1130.

30. Kandeel AA, Zeid M, Helm T, Lillie MA, Donahue E, Ambrus JL Jr. Evaluation of chronic urticaria in patients with Hashimoto thyroiditis. J Clin Immunol. 2001; 21:335–347.
31. Levy Y, Segal N, Weintrob N, Danon YL. Chronic urticaria: association with thyroid autoimmunity. Arch Dis Child. 2003; 88:517–519.

32. Aversano M, Caiazzo P, Iorio G, Ponticiello L, Laganá B, Leccese F. Improvement of chronic idiopathic urticaria with L-thyroxine: a new TSH role in immune response? Allergy. 2005; 60:489–493.

33. Nuzzo V, Tauchmanova L, Colasanti P, Zuccoli A, Colao A. Idiopathic chronic urticaria and thyroid autoimmunity: Experience of a single center. Dermatoendocrinol. 2011; 3:255–258.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download