Abstract
Background
Laser/light-based devices may provide an alternative to conventional acne therapeutics in some patients with nonresponsive acne.
Objective
We investigated the efficacy of red or infrared light-emitting diode (LED) devices in a mouse model of Propionibacterium acnes-induced inflammation through clinical examination and histopathological and immunohistochemical studies.
Methods
A human-derived Propionibacterium acnes suspension (109 colony-forming units /µl) was injected into the back of an HR-1 mouse. Then, a 28.9 J/cm2 650-nm red LED or 9.3 J/cm2 830-nm infrared LED was applied to the mouse with P. acnes-induced inflammation once daily for 2 weeks. Two weeks after treatment, histological findings with hematoxylin and eosin staining and expression levels of inflammatory biomarkers (integrin α6, neutrophils, interleukin [IL]-1β, and matrix metalloproteinase [MMP]-2/9) were evaluated in tissue specimens using immunohistochemical staining.
Results
Mice treated with red and infrared LED showed clinical improvement in inflammatory nodules compared to mice in the control group. Red LED was much more effective than infrared LED. Epidermal hyperplasia, comedone-like cysts, and integrin α6 expression improved to a similar extent in the red and infrared LED treatment groups and control group. Neutrophil, IL-1β, MMP-2, and MMP-9 expression after treatment with red and infrared LED decreased considerably compared to expression in the control group.
Acne is a very common skin disorder occurring in the follicular infundibulum12. Propionibacterium acnes colonization in the follicular infundibulum is a major cause of acne and plays an important role in inducing an inflammatory event3. In addition, P. acnes can cause abnormal differentiation and proliferation in epidermal keratinocytes1. Therefore, acne animal models can be established using P. acnes. The establishment of animal models for acne is helpful to expand research fields and to develop new therapeutic modalities. Although various animal models for acne such as the Mexican hairless dog and the Rhino mouse exist, an elucidative model is still needed4. Our animal model of inflammatory events was established with the injection of P. acnes into the back of an HR-1 mouse.
Laser/light-based devices may provide an alternative to conventional acne therapeutics in some patients. Many types of laser/light devices have been introduced to improve or cure inflammatory acne. Light-emitting diode (LED) devices are one of the most popular light-based devices for the treatment of acne. LED devices, especially those emitting blue and red light, directly or indirectly target P. acnes5.
In this study, we investigated the efficacy of red or infrared LED devices in a mouse model of P. acnes-induced inflammation through clinical examination, pathology, and immunohistochemical studies.
Six-week-old female Hos:HR-1 mice (HR-1; SLC Inc., Hamamatsu, Japan) were kept under conventional laboratory conditions (at 20℃~24℃ in a humidified atmosphere of 40%~60%) after 1 week of acclimation. P. acnes (ATCC 11828) were isolated from the pustular lesions of Korean patients with moderate inflammatory acne. P. acnes from post-log phase cultures were grown on brain heart infusion agar and harvested, heat killed (95℃, 5 min), and lyophilized prior to injection. A P. acnes suspension was prepared with a concentration of 109 colony-forming units/µl. With a 30-gauge needle, the P. acnes suspension was injected intradermally in 20-µl aliquots on both sides of the mouse back. Four mice in group A were injected with P. acnes and were not treated with red or infrared LED. Three mice in group B were treated with red LED after P. acnes injection, and three mice in group C were treated with infrared LED after P. acnes injection. The animal study protocol was approved by the Ethics Committee for animal studies at Kyoungpook National University, Korea (KNU 2014-0135).
After P. acnes injection, the mice were irradiated using a 28.9 J/cm2 650-nm red LED in group B and a 9.3 J/cm2 830-nm infrared LED in group C once daily for 2 weeks.
The degree of clinical inflammatory changes was evaluated using digital photography at baseline and at weeks 1 and 2 after P. acnes injection.
Tissue samples of mice were taken 2 weeks after P. acnes injection. Eight tissue samples were collected from group A, 6 tissue samples from group B, and 6 tissue samples from group C. Paraffin-embedded tissue sections of 3-µm thickness were processed for light microscopy. Hematoxylin and eosin (H&E) and immunohistochemical staining was performed using standard techniques. The primary antibodies were as follows: integrin α6 (diluted 1:150; Santa Cruz Biotechnology Inc., Dallas, TX, USA), neutrophils (diluted 1:80; Abcam, Cambridge, UK), interleukin (IL)-1β (diluted 1:150; Abcam), matrix metalloproteinase (MMP)-2 (diluted 1:300; Abcam), and MMP-9 (diluted 1:250; Abcam). The histological changes in each group were evaluated semiquantitatively with a 3-point scale: mild (+), moderate (++), and prominent (+++), with an emphasis on changes in inflammation, epidermal/follicular wall thickness, and formation of cystic structures containing keratinized plugs (microcomedone-like cystic structures) in the dermis. The degree of immunohistochemical staining in each group was also evaluated semi-quantitatively with a 3-point scale: mild (+), moderate (++), and dense (+++).
Tissue samples obtained from the three groups (A, B, and C) were stained with H&E. Stains from the treatment groups were compared to those of the control group without P. acnes injection. Improvement in epidermal hyperplasia and thickening in group B and group C was similar to that observed in group A (Fig. 2, Table 1). The number and size of the microcomedone-like cysts in the upper dermis above the focus of inflammation in group B and group C decreased to an extent similar to that in group A (Fig. 2, Table 1).
Currently, several animal models can be used for acne research. Among them, rabbit ears and Rhino mice have been used frequently to determine the compound comedogenicity of acne lesions67. However, the rabbit-ear and Rhino mice models have some limitations, including a lack of bacterial colonization and inflammation47. Therefore, these models are not helpful for inflammatory acne research. In addition, the use of rabbits may be inconvenient for large-scale drug screening and vaccinations. As proven by our previous study, P. acnes injection into the HR-1 mouse induces acneiform inflammatory nodules with overlying epidermal hyperplasia and superficial secondary microcomedones. Therefore, the HR-1 mouse is suitable for inflammatory acne research.
In this study, HR-1 mice were used to determine the efficacy of red or infrared LED treatment of inflammatory acne. Laser/light-based devices are an alternative to conventional acne therapeutics in some patients who experience side effects or recurrence with conventional treatments. Treatment with ultraviolet (UV) A and UVB was found to have a marginal beneficial effect for acne8. Blue light is most effective in the photoactivation of the endogenous porphyrin component of P. acnes, because the 407~420-nm wavelength light has the strongest porphyrin photoexcitation coefficient. However, blue light cannot access the deeper dermis9. On the contrary, red light penetrates deeper to the sebaceous glands and has anti-inflammatory properties, as apparent from its influence on cytokine release from macrophages1011. According to an action spectrum for the inactivation of P. acnes, the sensitivity of P. acnes is the highest with shorter wavelengths and decreases with increasing wavelength12. A combination of blue and red LED application appears to have excellent potential in the treatment of mild-to-severe acne13. The infrared laser light, at such strengths as 1,450 nm and 1,540 nm, selectively produces an injury zone in the dermis, where the sebaceous glands are located, sufficient enough to inhibit high sebum production, leading to acne improvement9.
In this study, both red and infrared LED had anti-inflammatory effects on HR-1 mice with inflammatory nodules composed mainly of neutrophils and histiocytes. Red LED was much more effective than infrared LED. A decrease in the expression of inflammatory markers, including neutrophils, IL-1β, MMP-2, and MMP-9, was shown in HR-1 mice after treatment with red or infrared LED. Their expressions after treatment with red LED decreased to a greater extent than those after treatment with infrared LED. However, the epidermal hyperplasia of the HR-1 mice after red or infrared LED treatment was similar to that after no LED treatment. The size and number of microcomedone-like cysts after red or infrared LED treatment were similar to those after no LED treatment. In addition, the expression of integrin α6 after red or infrared LED treatment was similar to that after no LED treatment. Unfortunately, a limitation to this study is that these animal models do not fully represent inflammatory acne.
In conclusion, red or infrared LED has the potential to relieve inflammatory acne and decrease the expression of inflammatory biomarkers; red LED is much more effective than infrared LED. HR-1 mice with P. acnes-induced inflammation represent a good model for the investigation of inflammatory acne.
Figures and Tables
Fig. 1
Clinical inflammatory lesions in group B and group C improved greatly compared to those in group A.
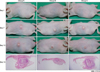
Fig. 2
Improvement in epidermal hyperplasia and microcomedone-like cysts in group B and group C was similar to that in group A.

Fig. 6
Matrix metalloproteinase (MMP)-2 and MMP-9 expression decreased greatly in group B and group C compared to that in group A.
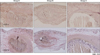
Table 1
Summary of the histological analysis and immunohistochemical profile results

ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2007017); Daegugyeong Institute for Regional Program Evaluation.
References
1. Shaheen B, Gonzalez M. Acne sans P. acnes. J Eur Acad Dermatol Venereol. 2013; 27:1–10.
2. Huh SY, Na JI, Huh CH, Park KC. The effect of photodynamic therapy using indole-3-acetic Acid and green light on acne vulgaris. Ann Dermatol. 2012; 24:56–60.

3. Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003; 121:20–27.

5. Mariwalla K, Rohrer TE. Use of lasers and light-based therapies for treatment of acne vulgaris. Lasers Surg Med. 2005; 37:333–342.

6. Nakano K, Kiyokane K, Benvenuto-Andrade C, González S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol Physiol. 2007; 20:29–36.

7. Nakatsuji T, Shi Y, Zhu W, Huang CP, Chen YR, Lee DY, et al. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to Propionibacterium acnes infection in vivo. Proteomics. 2008; 8:3406–3415.

8. Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC. Phototherapy in the treatment of acne vulgaris: what is its role? Am J Clin Dermatol. 2004; 5:211–216.
9. Elman M, Lebzelter J. Light therapy in the treatment of acne vulgaris. Dermatol Surg. 2004; 30:139–146.

11. Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers Surg Med. 1989; 9:497–505.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download