Abstract
Background
In cases of early stage subungual melanoma (SUM), conservative treatment with non-amputative wide excision of the nail unit and subsequent skin graft is preferred over amputation to preserve the involved digit.
Objective
We report a series of patients with SUM treated with conservative surgery and suggest an effective supplementary treatment process.
Methods
We retrospectively reviewed 10 patients (2 males, 8 females) who were diagnosed with in situ or minimally invasive SUM on the first biopsy and underwent non-amputative wide excision of the nail unit. All patients underwent secondary intention healing during the histopathological re-evaluation of the entire excised lesion, and additional treatment was administered according to the final report.
Results
In two of 10 patients, amputation was performed because of the detection of deep invasion (Breslow thickness: 4.0, 2.3 mm) from the final pathologic results, which differed from the initial biopsy. In six patients who received delayed skin graft, the mean total time required for complete healing after secondary intention healing and the skin graft was 66.83±15.09 days. As a result of this delayed skin graft, the final scarring was similar to the original shape of the nail unit, scored between 5 and 10 on a visual analogue scale. Most patients were satisfied with this conservative surgery except one patient, who had volar portion involvement and received an interpolated flap instead of a skin graft.
Subungual melanoma (SUM) accounts for approximately 20% of all melanomas in dark-skinned and Asian populations1. Since early skin biopsy of the nail is difficult, diagnosis of SUM is often delayed and they are usually diagnosed at advanced stages, resulting in poor prognosis23. On the basis of the rarity of SUM in Caucasian people and its poor prognosis, amputation was conventionally the treatment of choice for SUM into the 1980s and even 1990s; the main concern was determining how much of the finger to amputate depending on the tumor depth and ulceration456.
As the treatment of SUM by finger or toe amputation results in severe functional, esthetic, and psychological impairment7, conservative surgery without amputation has been attempted for SUM in situ or SUM with a low level of dermal invasion, showing favorable cosmetic and functional outcomes8910. In addition, conservative surgery does not worsen the prognosis of patients with minimally invasive SUM, although data are limited because of the rarity of SUM81112.
To perform this non-amputative procedure, it is a prerequisite that conservative surgery is sufficient for tumor clearance in the case of SUM. The nail unit has unique anatomical characteristics, and the invasion depth of the SUM varies according to the anatomical part1314. Therefore, the decision to perform conservative surgery on the basis of the first biopsy result carries a risk of incomplete resection.
We delayed reconstruction until we received the final histopathological report of the entire excised lesion, and then further amputation or reconstruction was decided on according to the report. Here, we report a series of SUM patients treated with this modality and propose an effective treatment protocol for SUM based on the clinical outcomes of these cases.
We retrospectively reviewed 10 patients (two males, eight females) who underwent non-amputative wide excision of the nail unit as the first-choice treatment for SUM from October 2006 to August 2011 at the Severance Hospital, Yonsei University Health System, Seoul, Korea. All patients were histopathologically diagnosed with SUM in situ or with a low level of dermal invasion (i.e., no evidence of bone invasion) upon the initial nail unit biopsy14. We reviewed each patient's medical records, including age, sex, onset location, clinical photos, treatment methods and duration, and the presence of complications. The study protocol was approved by the institutional review board of Yonsei University Severance Hospital (IRB No. 4-2013-0707) and was conducted according to the Declaration of Helsinki Principles.
The initial treatment for the patients was wide excision (Fig. 1). The safety margin was 0.5 cm from pigmented lesions in case of positive Hutchinson's sign, and 0.5 cm from the nail structure in case of negative Hutchinson's sign. For the deep margin, everything above the periosteum was removed. Histopathological re-examination was subsequently performed on the tissue removed en bloc. Each specimen was initially sliced along its longitudinal axis from the proximal nail fold to the hyponychium at 2-mm intervals, and histologic examinations of 4 sequential 4-µm-thick sections were performed. Tissue staining with hematoxylin and eosin, S-100 protein, HMB45, and Melan A was performed, and the Breslow thickness (from the lowest level of the nail plate to the bottom of the tumor) was recalculated.
During the histopathological re-evaluation, closed wound dressing was applied to the skin defect and changed every 3~4 days. At each visit, the wound was cleansed with 1% povidone iodine solution, and a non-adherent porous silicone wound contact layer (Mepitel; Mölnlycke Health Care, Gothenburg, Sweden) was applied directly to the wound, followed by a nonadherent absorbent contact layer made from polyurethane foam (Mepilex; Mölnlycke Health Care). Once in position, the dressing was held in place with a bandage or other suitable retention aid.
Depending on the final pathological report, amputation was performed if the melanoma extended into the deep margin, while delayed skin graft was performed if there was no extension. In patients who underwent skin graft, a sufficient secondary healing period was provided to ensure the growth of adequately thick granulation tissues to cover up the exposed bone and reduce the defect area for grafting. Full-thickness skin graft (FTSG), approximating the size and shape of the original nail, was conducted using a small piece of skin harvested from lower abdomen (Fig. 2).
To evaluate clinical outcomes, images of SUM patients treated with delayed skin graft were taken at the time of complete healing, and evaluations were performed by two individual dermatologists using a visual analog scale (VAS). The VAS is a 10-cm linear scale in which the left and right sides reflect the worst (score of 0) and best (score of 10) outcomes, respectively. In addition, patient satisfaction was evaluated at the end of treatment according to a 4-point grading scale (0=unsatisfied, 1=slightly satisfied, 2=satisfied, 3=very satisfied).
The mean age of the 10 SUM patients who received non-amputative wide excision of the nail unit as the first-choice treatment was 46.4 years (range, 18~76 years). Two out of 10 patients received amputation as an additional treatment, because the final pathologic result revealed deep invasion (case no. 1: Breslow thickness=2.3 mm; case no. 3: Breslow thickness=4.0 mm) in the completely excised lesion (Table 1). Although no deep invasion was observed in case no. 2, amputation was performed because the patient complained of severe pain and the skin graft was expected to be difficult due to a skin defect involving the volar portion after wide excision. In another case of volar portion involvement (case no. 4), an interpolated flap was performed using the skin of the adjacent fingers to restore the skin defect (Fig. 3). The other six patients' skin defects were restored by FTSG after confirming tumor clearance. Recurrence was not observed in any patient during follow-up period (range, 10~69 months).
In the six patients who received FTSG, the time required for secondary healing before the skin graft was 35.83±6.71 days (mean±standard deviation) on average, and the mean duration required for complete healing after skin graft was 31.00±12.28 days (range, 16~49 days). However, in case no. 4 who received interpolated flap, the time required for secondary healing was 59 days, and the period required for complete healing was 91 days. Furthermore, the patient complained of intermittent pain during this period (Table 2).
As a result of delayed skin graft after pathologic re-evaluation, final scarring was similar to the original shape of the nail unit, and VAS scores ranged from 5~10 (mean, 6.25±1.48). Almost all patients were satisfied with this surgery protocol, scoring 2 (satisfied) to 3 (very satisfied) on the 4-point grading scale, except the patient of case no. 4, who had volar portion involvement and received an interpolated flap (Table 2).
The invasion depth of SUM may vary depending on the special parts of the nail, such as the nail matrix, nail bed, and hyponychium, owing to the anatomical nature of nails313. While these special characteristics of SUM should be considered in the staging of melanoma and selecting an appropriate treatment method, even recent treatment guidelines do not present clear instructions for SUM treatment, possibly because SUM is rare among Caucasians15161718. In addition, various conservative surgeries that have recently been attempted to treat SUM in situ or in SUM with minimal invasion may have a risk of incomplete resection if repair is performed immediately after wide excision without pathological re-examination of the overall excised lesion.
In two of 10 cases in the present series, in contrast to the initial histological findings, deep invasion of SUM was identified with Breslow thicknesses of 4.0 mm and 2.3 mm in two cases by the final histopathological re-evaluation of the entire tumor, and amputations were performed. In particular, we administered adjuvant interferon for case no. 3, whose final result also showed neurotrophism of neoplastic melanocytes, which has a high risk of local recurrence19. Therefore, our delayed treatment process has the advantage of clearing the tumor through the re-evaluation of the completely excised lesion.
Another important feature of our treatment process is that secondary intention healing was applied to restore the skin defect of the nail unit during the period required for pathological re-examination. Several reports suggest secondary healing is better than skin grafting for fingertip amputation, resulting in good restoration of the original soft tissue thickness and sensation in the injured area20212223. In the skin defects of our patients, remarkable restoration of the nail folds and hyponychium was achieved with secondary intention healing during the re-evaluation period, almost to a degree that could be considered regeneration. In addition, sufficient granulation tissue was allowed to grow over the exposed bone before the skin graft, and its abundant blood supply may have been the reason for the excellent survival of the grafted skin. As the size and shape of the skin graft matched that of the original nail, the contracted grafted skin resembled a nail, making it more cosmetically acceptable than a smooth fingertip without a nail. For these reasons, VAS scores of SUM patients treated with delayed skin graft were content, and the majority of patients were also satisfied with their conservative surgery.
The drawbacks of our treatment process include the time required for the final determination of complete tumor removal and the prolonged treatment period for sufficient granulation tissue formation, because SUM is treated in two sessions. In particular, if the scope of wide excision involves the volar portion, problems such as severe pain, prolonged healing time, etc., may occur during the treatment course. In case no. 4, which was treated with an interpolated flap on the skin defect of the volar portion, the patient complained of intermittent pain and took longer to heal completely than patients who received skin graft (91 vs. 31±12.28 days). Therefore, sufficiently explaining the treatment process to patients is important, and the patient's opinion should play a role in treatment selection.
In conclusion, although there were too few cases to analyze the effects of non-amputative wide excision of the nail unit and delayed repair on patient survival rate, the present findings support the fundamental oncologic principle of "tumor clearance first, reconstruction second." Thus, this delayed approach could significantly reduce the risk of incomplete resection and improve cosmetic outcomes.
Figures and Tables
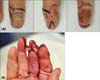
Fig. 2
Images of clinical progress. (A) Case no. 9. An 18-year-old female patient was diagnosed with melanoma in situ from the initial skin biopsy. (B) Excision with a 0.5-cm-wide margin was performed, and tumor clearance was subsequently confirmed by histopathological re-evaluation in the entire excised lesion. (C) The deep margin was bone contact. (D) Granulation tissues were sufficiently formed during a secondary healing period of 34 days after wide excision. (E) Skin graft was subsequently performed. (F) Complete healing was observed 16 days after skin graft.

Fig. 3
Clinical images of case case no. 4 (female/76). (A) Wide excision was performed with a 0.5-cm safety margin from the pigmented lesion and nail structure. (B) Two months later, an interpolated flap was made using the skin of the adjacent fingers to restore the skin defect.
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No.2013R1A2A2A04015894).
References
2. Rex J, Paradelo C, Mangas C, Hilari JM, Fernández-Figueras MT, Ferrándiz C. Management of primary cutaneous melanoma of the hands and feet: a clinicoprognostic study. Dermatol Surg. 2009; 35:1505–1513.
3. Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, Quinn MJ, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007; 31:1902–1912.
4. Park KG, Blessing K, Kernohan NM. The Scottish Melanoma Group. Surgical aspects of subungual malignant melanomas. Ann Surg. 1992; 216:692–695.

5. Quinn MJ, Thompson JE, Crotty K, McCarthy WH, Coates AS. Subungual melanoma of the hand. J Hand Surg Am. 1996; 21:506–511.

6. Heaton KM, el-Naggar A, Ensign LG, Ross MI, Balch CM. Surgical management and prognostic factors in patients with subungual melanoma. Ann Surg. 1994; 219:197–204.

7. Merle M. Reconstruction of amputated thumb: 20 years of development of techniques and indications. Bull Acad Natl Med. 1996; 180:195–210. discussion 211-214
8. Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. Br J Dermatol. 2011; 165:852–858.

9. Clarkson JH, McAllister RM, Cliff SH, Powell B. Subungual melanoma in situ: two independent streaks in one nail bed. Br J Plast Surg. 2002; 55:165–167.

11. Nguyen JT, Bakri K, Nguyen EC, Johnson CH, Moran SL. Surgical management of subungual melanoma: mayo clinic experience of 124 cases. Ann Plast Surg. 2013; 71:346–354.
12. Moehrle M, Metzger S, Schippert W, Garbe C, Rassner G, Breuninger H. "Functional" surgery in subungual melanoma. Dermatol Surg. 2003; 29:366–374.

13. Izumi M, Ohara K, Hoashi T, Nakayama H, Chiu CS, Nagai T, et al. Subungual melanoma: histological examination of 50 cases from early stage to bone invasion. J Dermatol. 2008; 35:695–703.

15. Marsden JR, Newton-Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. British Association of Dermatologists Clinical Standards Unit. Revised U.K. guidelines for the management of cutaneous melanoma 2010. Br J Dermatol. 2010; 163:238–256.

16. Bichakjian CK, Halpern AC, Johnson TM, Foote Hood A, Grichnik JM, Swetter SM, et al. American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011; 65:1032–1047.

17. Coit DG, Andtbacka R, Anker CJ, Bichakjian CK, Carson WE 3rd, Daud A, et al. National Comprehensive Cancer Network (NCCN). Melanoma, version 2. 2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013; 11:395–407.
18. Coit DG, Andtbacka R, Bichakjian CK, Dilawari RA, Dimaio D, Guild V, et al. NCCN Melanoma Panel. Melanoma. J Natl Compr Canc Netw. 2009; 7:250–275.

19. Baer SC, Schultz D, Synnestvedt M, Elder DE. Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer. 1995; 76:2242–2247.

20. Hoigné D, Hug U, Schürch M, Meoli M, von Wartburg U. Semi-occlusive dressing for the treatment of fingertip amputations with exposed bone: quantity and quality of soft-tissue regeneration. J Hand Surg Eur Vol. 2014; 39:505–509.

21. de Boer P, Collinson PO. The use of silver sulphadiazine occlusive dressings for finger-tip injuries. J Bone Joint Surg Br. 1981; 63B:545–547.

22. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993; 18:416–422.

23. Muneuchi G, Tamai M, Igawa K, Kurokawa M, Igawa HH. The PNB classification for treatment of fingertip injuries: the boundary between conservative treatment and surgical treatment. Ann Plast Surg. 2005; 54:604–609.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download