Abstract
Spitzoid melanoma is a subtype of melanoma that, clinically and histologically, resembles a Spitz nevus. Clinically, spitzoid melanomas usually evolve from amelanotic nodular lesions, growing to 1 cm or more in diameter. They often remain clinically undiagnosed because of their wide variety of clinical appearances and a lack of pigmentation. Distinguishing a Spitz nevus from a spitzoid melanoma can be extremely difficult. Features that favor the diagnosis of a spitzoid melanoma are asymmetrical shape, diameter greater than 1 cm, a lesion with a deep invasive component, and a high degree of cytologic atypia. There have been only rare reports in the literature of the presence of giant cells in malignant melanoma, and the presence of these cells may result in its misdiagnosis as a histiocytic tumor. We present a case of spitzoid melanoma on the right ankle of a 22-year-old-woman.
Spitzoid melanoma is a subtype of melanoma that clinically and histologically resembles a Spitz nevus1. Almost all Spitz tumors occur in patients younger than 20-year-old. The older patients, particularly those older than 20 or 30 years old, have a greater likelihood of malignancy2. Clinically, spitzoid melanomas are usually changing amelanotic nodular lesions, and can grow to a diameter of 1 cm or more1. They often go clinically undiagnosed because of their wide range of clinical appearances and a lack of pigmentation.
Spitz tumors commonly affect the extremities and face, but atypical Spitz tumors may also, less frequently, affect other areas such as the back2. They may resemble hemangiomas, pyogenic granulomas, xanthogranulomas, and basal cell carcinomas1.
Distinguishing between a Spitz nevus and a spitzoid melanoma can be extremely difficult. Features that support the diagnosis of a spitzoid melanoma are asymmetric shape, diameter greater than 1 cm, a lesion with a deep invasive component, and a high degree of cytologic atypia3. Here, we present a case of amelanotic spitzoid melanoma on the right ankle of a 22-year-old woman.
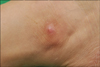
A 22-year-old female was referred to our clinic complaining of an asymptomatic nodule on the right ankle. She had a single erythematous bean-sized nodule on her right shin and an excisional biopsy was performed. The skin lesion had been present since she was 2 years old. On physical examination in our clinic, there was no other obvious lesion apart from the skin lesion. Examination of the skin revealed a single, relatively ill-defined, 1.5×1 cm-sized erythematous nodule on the lateral aspect of the right ankle (Fig. 1). There was no indication of trauma or irritation on the lesion. Laboratory examinations comprised a complete blood count, a blood coagulation test, routine chemistry, and a venereal disease research laboratory test; all results were within the normal range or negative. A skin biopsy from the lesion showed nodules and sheets of epithelioid tumor cells with surrounding chronic inflammation in the dermis. There was no prominent junctional activity, in either the slide from the first cut or that from a deeper shave. There was no obvious nest of conventional nevus cells and there were no conspicuous melanin granules in the specimen (Fig. 2A). In a high power field, the tumor cells were multinucleate and very pleomorphic. These cells had abundant cytoplasm and large vesicular nuclei, which contained prominent eosinophilic nucleoli (Fig. 2B, C). The multinucleated giant cells were dispersed throughout the whole dermis. The tumor cells had invaded the deep dermis; there was no intravascular or perineural invasion of the tumor cells. Breslow thickness was 2.8 mm and there was no tumor cell invasion of the inferior and lateral resection margins. The mitotic rate was 3/mm2 in the most mitotically active area. Immunohistochemical detection for S100 and Melan-A was positive in the tumor cells, while it was negative for HMB-45. Cytokeratin, CD31, CD34, CD68, and EMA staining were also negative (Fig. 3A, B, Table 1). Based on the clinical and histological findings, the patient was diagnosed with a spitzoid melanoma. She was referred to the department of oncology for evaluation of possible distant metastasis, which showed a negative result. Without any additional treatment, it was decided that the patient would be kept under close observation. After informing her of the risks of recurrence, we have been following up with this patient for 3 years, and no evidence of recurrence has been seen since the excision.
Spitzoid melanomas usually present as amelanotic nodules, often reaching 1 cm or more in diameter1. The clinicopathologic classification and diagnosis of Spitz nevus, and its distinction from melanoma, are some of the most difficult topics in dermatopathology. Older patients, particularly those older than 20 or 30 years of age, have a greater likelihood of malignancy2. Features supporting a diagnosis of spitzoid melanoma include: a large size (exceeding 10 mm in diameter); ulceration; deep dermal penetration; asymmetry; a lack of circumscription; thinning of the epidermis; extensive, lateral pagetoid extension; an absence of Kamino bodies; a high degree of cytologic atypia; a high mitotic rate especially in the deep dermis; atypical mitoses; increased nucleus-to-cytoplasm ratio; vacuolated irregular cytoplasm; and prominent eosinophilic nucleoli3. In the presented case, the lesion had a 15 mm diameter, and histopathology showed deep dermal penetration, lack of circumscription, cytological atypia, and prominent eosinophilic nucleoli. These features supported the diagnosis of a spitzoid melanoma. Spitzoid melanomas can evolve de novo or they can be associated with a preexisting Spitz nevus. In our case, the patient had had the skin lesion since she was 2 years old. This patient's history suggests that the melanoma was associated with a preexisting Spitz nevus, not developed de novo. However, the incidence rate of melanoma evolving in Spitz nevi is currently unknown1. Requena et al.3 reviewed 18 cases of spitzoid melanoma. They identified 5 previously undefined histological patterns of spitzoid melanoma: "genuine", "uniform", "packed", "polypoid" and "pigmented". The genuine type involves a small compound spitzoid neoplasm with an ovoid nest of epithelioid and fusiform melanocytes. The uniform type is a predominantly intradermal spitzoid neoplasm composed of a sheet of epithelioid cells. The packed type is a predominantly intradermal spitzoid neoplasm with a scarce junctional component and a very compact nest with an artifactual cleft. The polypoid spitzoid melanoma is composed mainly of an intradermal spitzoid neoplasm in a polypoid configuration. The pigmented type shows striking amounts of melanin and melanophages3. Our patient's specimen showed an intradermal sheet of epithelioid melanocytes, decreased collagen bundles, and no artifactual cleft. These findings were consistent with the features of a uniform spitzoid melanoma.
A variety of multinucleated giant cells may be found in tumors of the skin, the most characteristic of which are Touton, floret-type, wreath-like, glassy, osteoclast-like, and pleomorphic4. Wreath-like giant cells contain hyperchromatic, peripherally located nuclei surrounded by an eosinophilic cytoplasm. This type of giant cell is occasionally found in melanocytic lesions, including melanoma, and was also found in our case. It is also well known that multinucleated giant cells are not uncommon in Spitz tumors.
Occasionally, melanomas are exceedingly pleomorphic, containing tumor giant cells with enlarged nucleoli and conspicuous, frequently abnormal, mitotic figures. These are the so-called "giant cell melanomas"5. These variants must sometimes be distinguished from metastatic tumors, including pleomorphic secondary carcinoma, sarcomas, and even anaplastic CD30+ lymphoma5. Multinucleated giant cells have been described in desmoplastic melanomas6 and lentigo maligna7. They tend to display more vesicular nuclei and prominent nucleoli8. The stimulus for their development is unclear.
In cases with atypical histopathology, it may be difficult to differentiate spitzoid melanoma from other immature tumors. In such cases, immunohistochemistry should be applied. In our case, immunohistochemical tests for S-100, HMB-45, Melan-A, cytokeratin, vimentin, CD31, CD34, CD68, and epithelial membrane antigen were performed. We found that the specimen stained positive for S-100, Melan-A, and vimentin. With this immunohistochemical profile, we could clearly delineate the lesion as a melanoma and not another mesenchymal tumor.
In summary, recognition of this rare form of melanoma, with its unusual morphology, is important to avoid a misdiagnosis, such as cutaneous histiocytic neoplasm. The lack of pigmentation meant that we were not initially suspicious of a possible melanoma, but the immunohisto-chemical studies contributed greatly to its diagnosis. Long-term follow-up and more case reports are needed to assess the prognostic implication associated with this unusual morphology.
Figures and Tables
Fig. 2
(A) Epithelial granuloma-like tumor in the dermis. Atypical mitosis was found predominantly on the deep dermis, but also a little on the superficial dermis. Kamino bodies and pagetoid spread were absent from the epidermal component. The deep dermal invasion pattern of the lower border showed the mixed type, both expansive and infiltrative types (H&E, ×40). (B) The tumor cells were multinucleate and very pleomorphic with abundant cytoplasm and large vesicular nuclei (H&E, ×200). (C) Large vesicular nuclei containing prominent eosinophilic nucleoli (H&E, ×400).

References
3. Requena C, Botella R, Nagore E, Sanmartín O, Llombart B, Serra-Guillén C, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with spitz nevus. Am J Dermatopathol. 2012; 34:478–486.

4. Hornick JL. Practical soft tissue pathology: a diagnostic approach. 1st ed. Philadelphia: Elsevier;2013. 279–296.
5. Calonje JE, Brenn T, Lazar AJ, McKee PH. McKee's pathology of the skin. 4th ed. Philadelphia: Elsevier;2012. p. 1221–1267.
6. From L, Hanna W, Kahn HJ, Gruss J, Marks A, Baumal R. Origin of the desmoplasia in desmoplastic malignant melanoma. Hum Pathol. 1983; 14:1072–1080.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download