Abstract
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma, and it rarely exhibits predilection for hair follicle and eccrine gland infiltration. Here, we present 2 similar cases that display folliculotropism with varying amounts of follicular mucinosis, with and without syringotropism. The features observed in both cases were cystic, comedo-like, acneiform lesions; generalized involvement with loss of body hair; pruritus; and hidradenitis suppurativa-like lesions. Hypohidrosis as well as nail and palmoplantar involvement with lichen planopilaris-like clinical features were unique characteristics of the first case. Despite the well-known aggressive behavior of follicular mycosis fungoides, the presented cases had a subtle, slowly progressive, but persistent, clinical course. Folliculotropic and syringotropic mycosis fungoides are variants of cutaneous T-cell lymphoma. Clinical presentations might be challenging, and multiple, deep biopsy specimens containing adnexal structures are required for this critical diagnosis. Aggressive treatment may not be necessary in cases having an indolent course, especially in those with syringotropism.
Folliculotropic mycosis fungoides (MF) is a rare variant of MF with neoplastic T-lymphocytes infiltrating the hair follicles, often sparing the epidermis1. According to the World Health Organization (WHO), when the eccrine glands are also infiltrated by neoplastic cells, the disease is designated as syringotropic MF1. This form is rarer, with <30 cases reported to date2. In the current guidelines, the syringotropic and folliculotropic forms are defined as a single clinical variant of MF1.
The clinical presentations of MF can vary. Involvement of the head and neck region is usually observed in folliculotropic forms, presenting with cystic, milia-like, and acneiform lesions1. Solitary, erythematous, punctate, and anhydrotic patches are observed in the syringotropic forms1.
Herein, 2 cases of folliculotropic MF are presented, with 1 case displaying syringotropism with unusual clinical findings. Despite the complexity of the clinical presentations, the cases had unexpectedly favorable outcomes.
A 50-year-old man presented with an 8-year history of severe itching, hypohidrosis, total loss of body hair, nail changes, acneiform lesions on the trunk, and scaly plaques on the scalp and palmoplantar regions (Fig. 1).
The patient's condition had been misdiagnosed as acne conglobate, and he had been treated with oral isotretinoin as well as oral and topical antibiotics for several years without any response. Soon after the loss of scalp hair and body hair, skin biopsy specimens were taken, and MF associated with follicular mucinosis was diagnosed (Fig. 2). Immunohistochemical examination showed that these atypical cells were CD4+ T-cells. He had been treated with oral methotrexate and cyclophosphamide-vincristine-adriamycin-prednisolone chemotherapy without any improvement of the skin lesions.
On dermatologic evaluation, the most relevant features were the pterygium formation observed in all the nails; palmoplantar involvement with atrophic erythematous scaly patches; purplish-brown linear macules on the axillary region; lichenoid, infiltrated, and purple papules on the trunk; and total loss of body hair. These findings prompted us to propose the diagnosis of lichen planopilaris. Comedo-like lesions on the entire scalp, face, and trunk; cystic and acneiform inflammatory lesions on the trunk; and scaly atrophic erythematous patches on the scalp were also observed. There was no lymphadenopathy or hepatosplenomegaly.
Biopsy specimens taken from the comedo-like lesions on the trunk, erythematous patches on the palmar and plantar regions, and purple macules on the axillary region revealed pilotropic cutaneous T-cell lymphoma without follicular mucinosis. Atypical cells comprising CD4+ T-lymphocytes could be observed within the follicle epithelium (Fig. 3). Sweat glands and ducts were also infiltrated by these atypical lymphocytes displaying the CD4+ phenotype (Fig. 4). Immunohistological examination and T-cell receptor β-chain gene rearrangement analysis of the skin biopsy specimens showed a clonal population of T-cells. Skin cultures taken from the pustules revealed the presence of Staphylococcus aureus. Routine laboratory investigations for systemic involvement, such as complete blood count, peripheral blood smear examination, and blood biochemistry, were unremarkable. Results of whole-body computed tomography were normal. The patient had no systemic involvement.
The patient was administered with 25 mg/d of acitretin with psoralen and ultraviolet A (PUVA) therapy thrice every week for 4 months along with a short-term oral antibiotic therapy (1,000 mg amoxicillin clavulanate, twice a day, 10 days) for staphylococcal superinfection. Significant improvement of skin lesions and relief of itching were observed at the end of 4 months. No atypical lymphocytes were observed on control biopsy specimens. Although PUVA therapy had been planned to be continued for residual clinical disease, the patient refused any further treatment. He has been followed-up for 7 years, exhibiting stable skin disease and no systemic involvement. No comorbid hematologic malignancy was observed.
A 57-year-old man presented with acneiform lesions that he had since childhood and loss of body hair and scalp hair. Dermatological examination revealed loss of body hair, eyelashes, and eyebrows, and some uninvolved scalp hairs. Generalized comedo-like lesions were observed prominently on the head and trunk. Cystic lesions were more prominent on the ears and scalp, and hidradenitis suppurativa-like lesions were observed on the axillary and inguinal regions (Fig. 5). Biopsy specimens taken from cystic lesions on the left arm revealed folliculotropic MF with follicular mucinosis (Fig. 6). Immunohistochemical analysis showed that the intraepithelial and perifollicular infiltrate mainly comprised CD3+ and CD4+ lymphocytes, with few CD8+ cells.
No clinically palpable lymphadenopathy or systemic involvement was detected. Routine laboratory investigations for systemic involvement were unremarkable. This patient also received 25 mg/d of acitretin with PUVA therapy thrice every week for 4 months. No atypical lymphocytes were observed on control biopsy specimens. No comorbid hematologic malignancy was present. Modest clinical improvement and relief of itching was observed. The patient has been followed-up for 6 years, and had not experienced any systemic involvement. No improvement in body hair was observed, but the patient has been relieved of itching.
Folliculotropic MF is usually hard to diagnose both clinically and histopathologically. Therefore, in cases with slight follicular involvement, findings of eccrine gland involvement could be helpful in the diagnosis3. According to the few studies reported on patients with folliculotropic MF, the percentage of accompanying eccrine gland involvement is 4% to 33% (Table 1)3,4,5,6,7,8,9,10. It is unclear whether folliculotropism or syringotropism is a function of specific cell-surface antigens of the T-cells, adhesion molecules, or an abnormality of follicular or syringeal epithelium2.
Besides having acneiform lesions, our presented cases had their own unique features-generalized hypohidrosis, hidradenitis suppurativa-like lesions, loss of all body hair, lichen planopilaris-like features, involvement of the nails as well as palmar and plantar regions, and a benign clinical course.
The main histological feature differentiating follicular MF from acne lesions is the infiltration of the hair follicle epithelium by small to medium, sometimes large, cerebriform cells showing a T-helper phenotype. The collection of acid mucopolysaccharides within the involved follicle may be another clue, if presented as observed in our cases. On the other hand, follicular cystic changes, hyperplasia of the follicular epithelium, and granulomatous inflammatory reaction secondary to ruptured hair follicles, reminiscent of acneiform lesions, may also be detected in follicular MF.
Generalized hypohidrosis is not a commonly reported feature of MF in any form; focal anhydrotic patches have been defined in syringotropic MF cases10,11,12,13. The prominent syringeal involvement observed may alter eccrine gland function, and it might be the reason for the generalized hypohidrosis observed.
Colonization of the follicles with a hidradenitis suppurativa-like clinical presentation was observed in both of the presented cases. S. aureus colonization is usually observed in patients with MF14.
Case 1 showed features such as lichen planus and lichen planopilaris. Purple, pruritic papules, and brownish purple patches were observed on the intertriginous regions. Gerami et al.8 have reported 2 cases having histologically interface dermatitis with lichen planopilaris-like pattern.
Palmoplantar involvement is another rare presentation of MF. According to Kazakov et al.15 and Resnik et al.16, the prevalence of palmoplantar MF is reported to be 0.6%, which is usually observed in forms that are more extensive. The affected skin shows mostly hyperkeratosis, and the clinical presentation is usually palmoplantar keratoderma. Therefore, some cases have been misdiagnosed as chronic palmoplantar eczema16,17. Punctate erythema, which is accepted as a characteristic feature of eccrine gland involvement, is also rarely observed on the palms and soles16.
The pterygium formation observed in Case 1 is a unique finding. According to the present literature, involvement of most of the nails is usually observed and presents as onychomadesis, onychodystrophy, onycholysis, hyperkeratosis, and yellow nail syndrome18.
There are some controversies on the success of phototherapy for syringotropic and folliculotropic MF19. Narrow-band ultra violet B (UV-B) treatment is inadequate because UV-B cannot penetrate into deep adnexal structures. This leaves a deep residual disease even when the epidermal component may seem to improve. The presented cases responded well to a combination of acitretin and PUVA treatment.
According to a WHO/EORTC consensus report, the prognosis of folliculotropic MF is worse than that of a tumor stage of classical MF20. The Dutch group reported 5- and 10-year disease survival rates of folliculotropic MF of 68% and 26%, respectively6.
However, the prognoses of cases of syringotropic MF differ from that of the folliculotropic forms. Reported cases of syringotropic MF, with only skin involvement, usually had a good prognosis. The 10-year survival rates was 83% to 97%, similar to that of the chronic forms of syringotropic MF13. Therefore, the stable course of Case 1 might be due to the syringeal involvement observed.
In conclusion, folliculotropic MF cases can be a diagnostic challenge because of the great variability in their clinical presentations. Persistent acneiform and cystic, comedo-like lesions observed in the elderly-especially when accompanied by alopecia and pruritus-should raise the suspicion of MF, and early multiple biopsy specimens should be taken for exact clinical diagnosis. Accompanying eccrine gland involvement could be the reason for unusual symptoms and clinical presentations such as hypohidrosis and palmoplantar involvement. The prognostic significance of syringotropism should be evaluated by performing further studies. Regardless of the clinical subtype, the treatment and follow-up of patients with MF should be performed by dermatologists to avoid unnecessary aggressive treatment protocols.
Figures and Tables
Fig. 1
(A) Acneiform papules, pustules, and comedo-like lesions on the chest of the patient in Case 1. (B) Nodulocystic lesions, acneiform papules, pustules, and comedo-like lesions located on the back of the patient in Case 1. (C) Total alopecia of the scalp and eyebrows, and comedo-like lesions located on the forehead of the patient in Case 1. (D) Lichenoid purple papules on the lateral chest wall of the patient in Case 1. (E) Purple lichenoid macules and patches with hyperpigmented borders on the axillary region with loss of axillary hair of the patient in Case 1. (F) Spiny hyperkeratotic 1 to 3-mm papules and erythematous scales on the soles of the patient in Case 1. (G) Scaly erythematous patches on the palms of the patient in Case 1. (H) Pterygium formation and anonychia on the nails of the patient in Case 1.
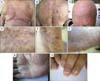
Fig. 2
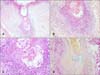
(A) Biopsy specimen taken from the scalp of the patient in Case 1. Mycosis fungoides associated with follicular mucinosis; band-like infiltration of atypical lymphocytes in the upper dermis, showing epidermotropism (H&E, ×100). (B) Biopsy specimen taken from the scalp of the patient in Case 1. Atypical lymphocytes within the hair follicle (H&E, ×200). (C) Biopsy specimen taken from the scalp of the patient in Case 1. Accumulation of mucopolysaccharides within the follicular epithelium (Alcian blue staining, ×200).

Fig. 3
Biopsy specimens taken from the trunk of the patient in Case 1. (A) Folliculocentric infiltration of atypical cells without follicular mucinosis. Note the enlargement of the follicular infundibulum with keratotic plugging (H&E, ×100). (B) CD4+ lymphocytes within the follicle epithelium (H&E, ×200).
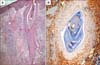
Fig. 4
Syringotropism: atypical lymphocytes within and around the eccrine glands observed in the biopsy specimen taken from the palm of the patient in Case 1 (H&E, ×200).
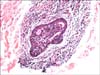
Fig. 5
(A) Loss of eyebrows and eyelashes; milia-like and comedo-like cystic lesions on the eyelids and forehead of the patient in Case 2. (B) Comedo-like lesions and alopecia of the vertex with some uninvolved scalp hair of the patient in Case 2. (C) Loss of body hair, and cystic lesions observed on the chest of the patient in Case 2. (D) Hidradenitis suppurativa-like lesions, comedones, and loss of axillary hair of the patient in Case 2.
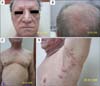
Fig. 6
Biopsy specimens taken from the left arm of the patient in Case 2. (A) Panoramic view, follicular dilatation, clear appearance of follicular epithelium, and dense perifollicular infiltrate (H&E, ×100). (B, C) Close-up view showing dense lymphocytic infiltrate, intraepithelial lymphocytes, and basophilic mucin accumulation (H&E; B: ×200, C: ×400). (D) Same follicle; mucin stains blue with colloidal iron. Plugging is also visible (colloidal iron staining, ×200).
References
1. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005; 105:3768–3785.

2. Yost JM, Do TT, Kovalszki K, Su L, Anderson TF, Gudjonsson JE. Two cases of syringotropic cutaneous T-cell lymphoma and review of the literature. J Am Acad Dermatol. 2009; 61:133–138.

3. Hitchcock MG, Burchette JL Jr, Olsen EA, Ratech H, Kamino H. Eccrine gland infiltration by mycosis fungoides. Am J Dermatopathol. 1996; 18:447–453.

4. Hodak E, Feinmesser M, Segal T, Yosipovitch G, Lapidoth M, Maron L, et al. Follicular cutaneous T-cell lymphoma: a clinicopathological study of nine cases. Br J Dermatol. 1999; 141:315–322.

5. Rongioletti F, Smoller B. The histologic value of adnexal (eccrine gland and follicle) infiltration in mycosis fungoides. J Cutan Pathol. 2000; 27:406–409.

6. van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002; 138:191–198.
7. Gómez-Diez S, Maldonado C, Fueyo A, Vázquez-López F, Fresno MF, Pérez-Oliva N. Folliculotropic mycosis fungoides. Study of four cases. Actas Dermosifiliogr. 2007; 98:486–490.

8. Gerami P, Rosen S, Kuzel T, Boone SL, Guitart J. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008; 144:738–746.
9. Lehman JS, Cook-Norris RH, Weed BR, Weenig RH, Gibson LE, Weaver AL, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010; 146:607–613.
10. Muniesa C, Estrach T, Pujol RM, Gallardo F, Garcia-Muret P, Climent J, et al. Folliculotropic mycosis fungoides: clinicopathological features and outcome in a series of 20 cases. J Am Acad Dermatol. 2010; 62:418–426.

11. Gerami P, Guitart J. The spectrum of histopathologic and immunohistochemical findings in folliculotropic mycosis fungoides. Am J Surg Pathol. 2007; 31:1430–1438.

12. Thein M, Ravat F, Orchard G, Calonje E, Russell-Jones R. Syringotropic cutaneous T-cell lymphoma: an immunophenotypic and genotypic study of five cases. Br J Dermatol. 2004; 151:216–226.

13. van Doorn R, Van Haselen CW, van Voorst Vader PC, Geerts ML, Heule F, de Rie M, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000; 136:504–510.
14. Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008; 159:105–112.

15. Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004; 18:397–415.

16. Resnik KS, Kantor GR, Lessin SR, Kadin ME, Chooback L, Cooper HS, et al. Mycosis fungoides palmaris et plantaris. Arch Dermatol. 1995; 131:1052–1056.

17. Topf S, Lüftl M, Neisius U, Brabletz T, Simon M Jr, Schuler G, et al. Mycosis fungoides palmaris et plantaris--an unusual variant of cutaneous T-cell lymphoma. Eur J Dermatol. 2006; 16:84–86.
18. Grande-Sarpa H, Callis Duffin KP, Florell SR. Onychodystrophy and tumor-stage mycosis fungoides confined to a single digit: report of a case and review of nail findings in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2008; 59:154–157.

19. Matsuoka Y, Yoneda K, Katsuura J, Moriue T, Nakai K, Sadahira C, et al. Successful treatment of follicular cutaneous T-cell lymphoma without mucinosis with narrow-band UVB irradiation. J Eur Acad Dermatol Venereol. 2007; 21:1121–1122.

20. Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. ISCL/EORTC. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007; 110:1713–1722.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download