Abstract
Apocrine carcinoma is a rare malignancy with invasive potential. It presents as painless, slow-growing, firm or cystic, red nodules with focal ulcerations. The tumor is capable of hematogenous dissemination to the liver, lungs, and bone as well as lymphatic spread. In addition, apocrine carcinomas cause intra-epidemial pagetoid spread. We report a case of an apocrine carcinoma related with extensive extramammary Paget's disease (EMPD). The relationship between apocrine carcinoma and EMPD remains to be understood. Co-existing cases with apocrine carcinoma and EMPD are discussed to better understand the relationship between these two malignant apocrine tumors.
The occurrence of apocrine carcinoma is very rare, and patients with this tumor do not exhibit any distinctive clinical features1. It usually presents as a single or a multi-nodular mass or as a plaque in the axillary or anogenital region; the size of tumor is 2~8 cm1. Apocrine carcinoma tumors are capable of hematogenous dissemination to visceral organs as well as lymphatic spread. However, pagetoid epidermal spread is uncommon in patients with apocrine carcinoma2. We report the case of a patient with apocrine carcinoma with extensive extramammary Paget disease (EMPD); the tumor showed aggressive local invasion and distant metastasis.
A 71-year-old man visited our hospital with a raised nodular mass of size 2×3×1 cm in his left groin and an erythematous area of 9×10 cm over the left groin (Fig. 1). He had first noticed the erythema and nodules 6 months before he visited our hospital. The erythema had gradually expanded in size. Ulceration with intermittent bleeding was noted within the nodular mass. On physical examination, edema of the left leg was observed, and lymph nodes in both inguinal regions were palpable. The results of routine hematological and biochemical examinations were normal. Examination of skin biopsies from the inguinal folds revealed features of EMPD in the epidermis and underlying glandular neoplastic foci in the reticular dermis and in the subcutaneous adipose tissue (Fig. 2A). Moreover, focal angiovascular invasion was noted. Typical Paget cells were scattered in the epidermis (Fig. 2B). The glandular neoplastic foci resembled carcinoma in situ. Some foci showed accumulation of secretion in central regions (Fig. 2C). The tumor cells exhibited abundant eosinophilic cytoplasm and large vesicular nuclei.
The tumor and Paget cells showed strong positive immunohistochemical staining for carcinoembryonic antigen (CEA), epithelial membrane antigen, cytokeratin 7 (CK7), and gross cystic disease fluid protein-15 (GCDFP-15) (Fig. 2D, F) but were negative for melanin-A, S-100, and CK20 (Fig. 2E). The patient was diagnosed with apocrine carcinoma related with extensive EMPD. Chest radiography, abdomino-pelvic computed tomography (CT), a gallium scan, a bone scan, and positron emission tomography-CT were performed to exclude the presence of internal malignancy. The results showed multiple lung, bone, and lymph node metastases. After consultation with a urologist and a surgeon, we concluded that this patient was inoperable, and opted for conservative treatment; however, the patient refused any treatment.
In rare cases, apocrine carcinoma co-exists with an overlying EMPD1. To our knowledge, only three cases in the groin, a relatively uncommon site for apocrine carcinoma, have been reported in the English literature3-5. The immunohistological staining patterns suggest that both EMPD and apocrine carcinoma are derived from the apocrine gland6,7. Subsequently, we postulated a relationship between EMPD and apocrine carcinoma. First, extramammary Paget cells are capable of progressing to invasive carcinoma and metastasizing to visceral organs; therefore, it is possible that invasive EMPD is the origin of apocrine carcinoma. Second, apocrine carcinoma involving the dermis exhibits intra-epidermal pagetoid spread and deep invasion into subcutaneous tissue. This characteristic is similar to that of mammary ductal adenocarcinoma that exhibits epidermotropic growth in the case of mammary Paget's disease. We think there is a greater likelihood of the second hypothesis, because the tumor mass on the upper dermis resembled carcinoma in situ. In addition, we observed some clustered apocrine carcinoma cells in the epidermis.
The histogenesis of EMPD is a controversial issue. Several investigators have proposed that EMPD is a heterologous entity. In some cases, EMPD represents a de novo adenocarcinoma that arises in the epidermis in situ, and in other cases EMPD represents epidermotropic metastasis or a direct extension of an associated internal malignancy8. In this regard, recent studies concerning perianal and vulvar EMPD have revealed cutaneous (primary) and endodermal (secondary) immunohistochemical subtypes of EMPD4. In the case of our patient, the immunohistochemical examination revealed a pattern similar to cutaneous EMPD; the tumor cells were positive for CK7 and GCDFP-15 but negative for CK20. That is, although an underlying apocrine carcinoma was present, the EMPD showed a cutaneous pattern. This result is different from that obtained in the case of endodermal EMPD with other underlying internal malignant tumors.
By reviewing three cases of apocrine carcinoma with an overlying EMPD that occurred in the groin, all four cases including our case presented with EMPD of the inguinal fold with a central nodule of apocrine carcinoma3-5. The immunohistochemical findings of the four cases are summarized in Table 1. Skin biopsies from the case of Bowling et al.5 showed EMPD in the inguinal fold, which stained positive for CK7. A biopsy from the papule showed invasive adenocarcinoma, which stained positive for the keratin markers MNF116, AE1, AE3, CK7, and CAM5.2, but was negative for S-100, CK20, prostate-specific antigen, prostate-specific acid phosphatase, and vimentin3. Interestingly, excision of the appendageal tumor was followed by complete resolution of the remaining EMPD. Hernandez and Copeland3 reported similar pathological results. That is, the tumor and Paget cells stained positive for CEA, AE1/3, and GCDFP-15 but negative for Melan-A, S-100, and CK20. In this case, they found lymph node infiltration of the adenocarcinoma. Finally, in the case of Pascual et al.4, Paget and dermal tumor cells were negative for S-100, HMB-45, and prostate-specific antigen but positive for periodic-acid-Schiff, CEA, CK7, CK20, GCDFP-15, mucin 1 and mucin2, human epidermal growth factor receptor2/neu and caudalrelated homeobox-2. Because of the CK20 positivity, they concluded that their case showed combined immunohistochemical findings suggestive of the cutaneous subtype and the endodermal subtype of EMPD with no associated internal malignancy.
In conclusion, we report a case of a patient who presented with apocrine carcinoma that possibly co-existed with EMPD in the groin. The results of this case study will facilitate the understanding of the relationship between these two kinds of malignant tumors.
Figures and Tables
Fig. 1
An erythematous plaque with an exophytic nodule on the left groin with lymphedema on the left leg.

Fig. 2
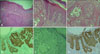
(A) Histopathology revealed features of extramammary Paget's disease in the epidermis and underlying glandular neoplastic foci in the reticular dermis as well as in the subcutaneous adipose tissue. (B) Large cells with large nuclei and abundant cytoplasm with some mitotic figures infiltrating the epidermis. (C) Apocrine carcinoma cells showing apocrine features; the tumor resembles a carcinoma in situ. (D) Positive immunostaining for cytokeratin 7 (CK7) in the tumor and Paget cells. (E) Tumor and Paget cells with negative immunostaining for CK20 (F) Positive reaction for gross cystic disease fluid protein-15 in the tumor and Paget cells (original magnification: A, ×40; B~F, ×100).
References
1. David W. David W, editor. Tumors of cutaneous appendages. Weedon's skin pathology. 2010. 3rd ed. Oxford: Elsevier Ltd;757–807.

2. Robson A, Lazar AJ, Ben Nagi J, Hanby A, Grayson W, Feinmesser M, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008. 32:682–690.
3. Hernandez JM, Copeland EM 3rd. Infiltrating apocrine adenocarcinoma with extramammary pagetoid spread. Am Surg. 2007. 73:307–309.

4. Pascual JC, Perez-Ramos M, Devesa JP, Kutzner H, Requena L. Extramammary Paget's disease of the groin with underlying carcinoma and fatal outcome. Clin Exp Dermatol. 2008. 33:595–598.

5. Bowling JC, Powles A, Nasiri N, Searle A, Bunker CB. Spontaneous regression of extramammary Paget's disease after excision of primary apocrine carcinoma, in an immunosuppressed patient. Br J Dermatol. 2005. 153:676–677.

6. Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget's disease--evidence for an apocrine origin An immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984. 8:43–50.
7. Miyamoto T, Inoue S, Adachi K, Takada R. Differential expression of mucin core proteins and keratins in apocrine carcinoma, extramammary Paget's disease and apocrine nevus. J Cutan Pathol. 2009. 36:529–534.

8. Krause W, Krisp A, Hörster S, Hoffmann R. Genital Paget's disease in a man. Eur J Dermatol. 2006. 16:75–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download