Abstract
Background
Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is a distinctive skin lymphoma characterized by neoplastic T-cell infiltration of the subcutaneous tissue, mimicking panniculitis.
Results
The mean patient age was 35 years (range: 7~73 years), with male predominance (2.5:1). Most patients presented with either nodules or plaques, occurring most commonly on the trunk, with two patients (14%) having hemophagocytic syndrome. Histopathologically, all patients showed infiltrates of small-to-medium pleomorphic cells mimicking panniculitis, with some also showing rimming, bean-bag cells, and fat necrosis. Most patients were positive for CD3 (14/14), CD8 (12/13), TIA-1 (9/9) and βf1 (5/5), but were negative for CD4 (11/12), CD20 (8/8), CD56 (14/14) and Epstein-Barr virus (8/8). Ten patients (71%) received chemotherapy and 2 (14%) died due to the disease, with an average survival time of 4 months. Survival analysis did not reveal any significant prognostic factors.
Subcutaneous panniculitis-like T-cell lymphoma (SPTL) is a distinctive skin lymphoma characterized by neoplastic cytotoxic T cell infiltration of the subcutaneous tissue, mimicking panniculitis. Two subtypes have been described, i.e., the SPTL of αβ T-cell and γδ T-cell origin, each of them showing a different phenotype and clinical course. T-cell receptor (TCR) αβ lymphomas are usually CD4-, and CD56-, but CD8+, and have an indolent course, whereas TCR γδ lymphomas are usually CD4-, and CD8-, but CD56+, are associated with a poor prognosis, and are often fatal due to the accompanying hemophagocytic syndrome1. The recent World Health Organization (WHO)-European Organization for Research and Treatment of Cancer (EORTC) classification of primary cutaneous lymphomas, however, restricted the category of SPTL to lymphomas expressing the TCR αβ phenotype, placing those expressing the TCRγδ phenotype into a new provisional category of cutaneous γδ T-cell lymphoma2. Few studies to date, however, have assessed patients with SPTL, as defined by the WHO-EORTC classification system. We therefore assessed the clinical and pathologic features of 14 Korean patients with SPTL, their response to treatment, and prognosis.
Twenty-two patients with SPTL were evaluated in the Dermatology Department of the Asan Medical Center, between January 1995 and September 2009. Eight patients were excluded owing to insufficient material or problems with the clinical data. The medical records of the remaining 14 patients were extensively reviewed, and patients' age, sex, clinical presentation, treatments, response to therapy and follow-up were recorded.
Representative histopathologic sections and immunohistochemical slides were reviewed by two dermatologists to identify the subcutaneous panniculitis-like T-cell lymphoma, according to the new WHO-EORTC classification. Additional staining was requested, if not previously performed. The antibodies used were directed against CD3 (polyclonal; Dako, Glostrup, Denmark), CD4 (1F6; Novocastra, Newcastle upon Tyne, UK), CD8 (C8/144B; Dako, Glostrup, Denmark), CD20 (L26; Dako, Glostrup, Denmark), CD30 (Ber-H2; Dako, Glostrup, Denmark), CD56 (123, C3; Zymed, South San Francisco, CA, USA), Granzyme B (GZB01; NeoMarkers, Fremont, CA, USA), TIA-1 (2G9; Dako, Glostrup, Denmark), and TCR-betaF1 (8A3; Thermo Scientific, Woburn, MA, USA).
ISH for EBV was performed on skin samples from eight patients, plus positive and negative controls, using the Ventana Benchmark autostainer and the Epstein-Barr Virus Early RNA (EBER) kit (Ventana Medical Systems, Tucson, AZ, USA).
Polymerase chain reaction (PCR) analysis of the TCR gamma gene was performed on routinely fixed, paraffin-embedded, tissue specimens from eight patients, as described previously3.
The overall survival was calculated from the date of histologically confirmed diagnosis until death or last follow-up. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed using the Statistical Product and Services Solutions (SPSS) software, version 12.0 (SPSS Inc., Chicago, IL, USA).
The clinical features of the 14 patients (10 male and 4 female) are summarized in Tables 1 and 2. The mean patient age was 35 years (range: 7~73 years), and the mean duration of skin lesions prior to diagnosis was 21.2 months.
All patients presented with multiple lesions, 10 with lesions on the trunk (71%), 9 with lesions on the lower extremities (64%), 8 with lesions on the upper extremities (57%) and 4 with lesions on the face (29%). Thirteen patients (93%) presented predominantly with nodules, 3 (21%) with plaque, and 2 (14% with swelling (Fig. 1); no lesion was accompanied by ulceration. Many patients showed evidence of extracutaneous manifestations at presentation. For example, of the 13 patients who underwent computed tomography (CT) scans, 4 (31%) had splenomegaly, including 2 (15%) with hepatomegaly, and 5 (39%) had lymphadenopathy. No patient had bone marrow involvement, as confirmed by biopsy. Two patients (14%) developed the hemophagocytic syndrome, and 9 of 13 patients (69%) presented with B symptoms, including fever, night sweats, and/or weight loss. Seven patients (50%) showed decreased white blood cell counts and increased liver enzyme concentrations, and six (43%) had anemia. Increased lactate dehydrogenase (LDH) concentrations were detected in 9 of 13 patients (69%). However, staging procedures showed no evidence of lymphoma outside the skin in any of the investigated 14 patients.
The clinical presentation of these patients included 3 with erythema nodosum, 2 with connective tissue disease, and one each with panniculitis, Sweet syndrome, hemophagocytic syndrome, and cellulitis.
The biopsy specimens of all 14 patients showed dense infiltrates, in a lobular pattern, of atypical lymphocytes, primarily in the subcutaneous tissue. In five patients, a small minority of lymphocytes extended into the deep dermis, in a periadnexal pattern in three and in a perivascular pattern in two.
The subcutaneous lesions consisted of pleomorphic, small-to-medium-sized cells, plus a few diffuse large T cells containing hyperchromatic, irregularly contoured nuclei (Fig. 2).
All 14 patients showed evidence of rimming (i.e., individual fat cells rimmed by atypical lymphocytes). A variable admixture of karyorrhexis was present, as were macrophages filled with nuclear debris in the cytoplasm, generating the typical bean-bag appearance. Thirteen patients showed evidence of necrosis, with five showing angiocentric infiltration, but none showed angioinvasion or angiodestruction.
In most patients, the neoplastic lymphocytes had a CD4-/CD8+ phenotype, except for one patient with a CD4+/CD8- phenotype. All tumors assayed were negative for CD20, CD30 and CD56. The cytotoxic proteins TIA-1 and granzyme B were expressed in 9 of 9 and 3 of 4 patients, respectively (Fig. 3). All five patients tested were positive for betaF1, confirming their alpha/beta T-cell phenotype. ISH showed that all eight patients assayed were negative for EBV. Four of eight patients showed clonal rearrangements of the TCR gamma gene.
Of the 14 patients, 6 were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy, 4 with chemotherapy other than CHOP, 3 with immunosuppressive therapy, and 1 with symptomatic therapy. Of the six patients receiving CHOP, two showed complete response (CR), one showed partial response (PR), and three showed progressive disease (PD). One patient died due to lymphoma after 4 months. Of the two patients achieving CR as initial response, one remained in CR at the time of follow-up. Of the five patients found to have disease during the follow-up, one patient with hemophagocytic syndrome (HPS) underwent autologous stem cell transplantation (ASCT) and radiotherapy.
Of the four patients receiving chemotherapy other than CHOP, one each had CR, PR, no response and PD. None attained complete remission during the follow-up period. After changing the chemotherapy regimen, one patient achieved CR during the follow-up.
Of the three patients treated initially with immunosuppressive agents (i.e., systemic steroids), including combinations of prednisone and hydroxychloroquine, only one achieved CR, whereas two showed PD, with one of the latter receiving chemotherapy (vorinostat). In addition, one patient with HPS received symptomatic treatment (i.e., transfusion, nutritional supplements, and diuretics), but died after 4 months.
Two patients underwent ASCT following chemotherapy. One patient was in CR, whereas the other had PD and was treated with radiotherapy.
At the end of the follow-up period, three patients had achieved CR and two patients had died. Survival analysis using the Kaplan-Meier method showed that the extent of skin involvement (localized vs multiple, p=0.290), the type of treatment (immunosuppressive therapy vs other, p=0.138), the presence of HPS (p=0.354), lymphadenopathy (p=0.193), splenomegaly (p=0.217), angiocentricity (p=0.170), the involvement of the dermis (p=0.153), and the positivity for TCR gamma gene rearrangement were not significantly related to the patient survival.
The recent WHO-EORTC classification of primary cutaneous lymphomas2 has restricted the category of SPTL to tumors expressing the TCR αβ phenotype, placing those expressing the TCR γδ phenotype into a new provisional category of cutaneous γδ T-cell lymphoma. There have been few studies of SPTL using this definition4,5. We therefore sought to determine the primary clinical, histologic and immunophenotypical characteristics of patients with SPTL and compare them with those of patients included in previous studies (Table 5).
In contrast to the previously reported female predominance, we observed a male predominance in our population. The mean patient age (35.1 years) was similar to that reported previously (i.e., 32.3 and 36 years)4,5. The mean disease duration prior to diagnosis was 21.2 months in our study. Most patients presented with a deep-seated plaque or nodule, most commonly in the trunk, but also in the lower extremities, upper extremities, and face. In contrast to previous findings, none of our patients had solitary lesions or ulcerations. B symptoms and splenomegaly were more common than previously reported, whereas the incidence of hemophagocytic syndrome was similar (14%) to that of a European study (17%).
SPTL has been associated with autoimmune disorders, including systemic lupus erythematosus, juvenile rheumatoid arthritis, type 1 diabetes mellitus, Sjogren syndrome, and idiopathic thrombocytopenic purpura4,6. The relationship between SPTL and lupus erythematosus profundus (LEP) is unclear. Histopathologic criteria have been found useful in differentiating LEP from SPTL, suggesting that they represent distinct entities7. Histopathologic criteria favoring the diagnosis of LEP include epidermal involvement, mucin depositions, the presence of reactive germinal centers, clusters of B cells or considerable number of admixed plasma cells, and polyclonal TCRγ gene rearrangement. In contrast, several features of LEP and SPTL have been found to overlap, suggesting that these patients should be categorized as having "atypical lymphocytic lobular panniculitis", distinct from both SPTL and LEP8. In a large European cohort of 63 patients with SPTL4, four patients were definitively diagnosed with LE and another four were initially misdiagnosed with lupus panniculitis. These findings indicate that features of SPTL and LEP may overlap in a small proportion of patients and that all patients with suspected SPTL should be screened for LE. In addition, SPTL may arise secondarily following long-standing LEP, suggesting that patients with LE, particularly those with LEP, should be monitored for development of SPTL9. Of our 14 patients, 1 showed overlapping features of LEP and SPTL, including mild vacuolar changes at the dermo-epidermal junction and a few plasma cells in the subcutaneous tissue. However, the presence of cellular atypia and clonal TCR gamma gene rearrangements, as well as the clinical characteristics, favored the diagnosis of SPTL. This patient was treated with immunosuppressive therapy (prednisone+HCQ). In contrast, none of our other patients showed any clinical, serologic or histopathologic abnormalities indicative of autoimmune disease.
TCR gene rearrangement was performed as an adjuvant diagnostic method in previously reported SPTL cases, with a different clonal detection rate, varying from 50% to 80%10,11. The presence of massive necrosis in the skin biopsy specimens may be a possible reason for cases with negative results12. In this study, clonal rearrangement of the TCR gamma gene was detected in 4 of 8 tested patients (50%), which was consistent with previous reports. In the patients who did not show clonal TCR gamma gene rearrangment, the diagnosis of SPTL had finally been made by histopathologic findings and immunophenotype, including the positive staining for TCR-beta F1.
SPTL is usually treated with combination chemotherapy, most commonly anthracycline-based agents, such as CHOP or CHOP-like regimens11. A recent European report4, however, suggested that prednisolone or other immunosuppressive agents may provide benefits equivalent to those of either CHOP-like or aggressive regimens, both in patients with primary tumors and those with relapsed disease.
We found that similar percentages of patients achieved CR following treatment with immunosuppressive (1 of 3 patients) and CHOP (2 of 6 patients) regimens. Patients with relatively indolent or localized disease tend to be treated with immunosuppressive therapy, but responses are relatively short-lived, with subsequent progression after steroid doses are tapered. Additional studies of immunosuppressive therapy in patients with SPTL are required before conclusions can be drawn.
ASCT has shown impressive response rates after high-dose chemotherapy, with most patients achieving CR at a median follow-up of 14 months13-16, although the conditioning regimens in these previous studies varied. Two of our 14 patients underwent ASCT following chemotherapy; although one was in CR, the other showed evidence of tumor recurrence.
SPTL has generally been associated with favorable prognosis and protracted disease course, with 5-year survival rates >80%10,11,17. Of our 14 patients, 2 died due to disease progression at a mean 4 months after diagnosis. Both the presence of HPS and angioinvasion have been found to be significant indicators of poor prognosis. HPS is a multisystem illness characterized by fever, wasting, adenopathy, hepatosplenomegaly, pancytopenia, coagulopathy, hyperferritinemia and hypertriglyceridemia, develops in about 20% of SPTL patients and is associated with a poor outcome, with a 5-year survival rate of 46%4. Two of our 14 patients had HPS, but none had angioinvasion. We did not identify any prognostic factors significant for survival. The extent of skin involvement (localized vs multiple), the type of treatment (immunosuppressive therapy vs other), the presence of HPS, lymphadenopathy, splenomegaly, angiocentricity, involvement of dermis, and the positive TCR gamma gene rearrangement were not significant prognostic factors.
This study had limitations. The follow-up period was relatively short to estimate the 5-year survival rate. Second, the number of included patients was small. Therefore, additional studies including larger number of patients and with a longer follow-up period are needed.
Figures and Tables
Fig. 1
Clinical findings in patients with subcutaneous panniculitis-like T-cell lymphoma. Shown are characteristic multiple erythematous indurated plaques and nodules on (a) the face of patient 16, (b) the trunk of patient 7, (c) the lower leg of patient 6, and (d) the upper extremities of patient 5.
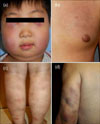
Fig. 2
Histopathologic findings in patients with subcutaneous panniculitis-like T-cell lymphoma. (a) Atypical lymphocyte infiltration in a lobular, panniculitis-like pattern (H&E, ×40). (b) Atypical lymphocytes rimming adipocytes (H&E, ×400). (c) Bean-bag cells (H&E, ×400). (d) Extensive necrosis (H&E, ×200).
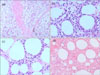
Fig. 3
Immunohistochemical analysis in patients with subcutaneous panniculitis-like T-cell lymphoma, showing that neoplastic cells in the subcutis were positive for (a) CD3, (b) CD8, (c) granzyme B, and (d) T-cell intracellular antigen-1 (all original magnification, ×200).
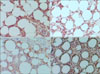
Table 2
Clinical manifestations of SPTL and follow-up data

SPTL: subcutaneous panniculitis-like T-cell lymphoma, HPS: hemophagocytic syndrome, LDH: lactate dehydrogenase, M: male, F: female, F: face, T: trunk, UE, LE: upper, lower extremities, CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone, ASCT: autologous stem cell transplantation, AW: alive and well, AWD: alive with disease, DWD: dead with disease, LN: lymph node involvement, BM: bone marrow involvement, CTX: chemotherapy, PD: prednisone, HCQ: hydroxychloroquine, RT: radiotherapy, CR: complete remission, PR: partial remission, SD: stable disease, PD: progressive disease.
References
1. Takeshita M, Okamura S, Oshiro Y, Imayama S, Okamoto S, Matsuki Y, et al. Clinicopathologic differences between 22 cases of CD56-negative and CD56-positive subcutaneous panniculitis-like lymphoma in Japan. Hum Pathol. 2004. 35:231–239.

2. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005. 105:3768–3785.

3. van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003. 17:2257–2317.

4. Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008. 111:838–845.

5. Kong YY, Dai B, Kong JC, Zhou XY, Lu HF, Shen L, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinicopathologic, immunophenotypic, and molecular study of 22 Asian cases according to WHO-EORTC classification. Am J Surg Pathol. 2008. 32:1495–1502.
6. Hahtola S, Burghart E, Jeskanen L, Karenko L, Abdel-Rahman WM, Polzer B, et al. Clinicopathological characterization and genomic aberrations in subcutaneous panniculitis-like T-cell lymphoma. J Invest Dermatol. 2008. 128:2304–2309.

7. Massone C, Kodama K, Salmhofer W, Abe R, Shimizu H, Parodi A, et al. Lupus erythematosus panniculitis (lupus profundus): clinical, histopathological, and molecular analysis of nine cases. J Cutan Pathol. 2005. 32:396–404.

8. Magro CM, Crowson AN, Byrd JC, Soleymani AD, Shendrik I. Atypical lymphocytic lobular panniculitis. J Cutan Pathol. 2004. 31:300–306.

9. Pincus LB, LeBoit PE, McCalmont TH, Ricci R, Buzio C, Fox LP, et al. Subcutaneous panniculitis-like T-cell lymphoma with overlapping clinicopathologic features of lupus erythematosus: coexistence of 2 entities? Am J Dermatopathol. 2009. 31:520–526.

10. Hoque SR, Child FJ, Whittaker SJ, Ferreira S, Orchard G, Jenner K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinicopathological, immunophenotypic and molecular analysis of six patients. Br J Dermatol. 2003. 148:516–525.

11. Massone C, Chott A, Metze D, Kerl K, Citarella L, Vale E, et al. Subcutaneous, blastic natural killer (NK), NK/T-cell, and other cytotoxic lymphomas of the skin: a morphologic, immunophenotypic, and molecular study of 50 patients. Am J Surg Pathol. 2004. 28:719–735.

12. Gallardo F, Pujol RM. Subcutaneous panniculitic-like T-cell lymphoma and other primary cutaneous lymphomas with prominent subcutaneous tissue involvement. Dermatol Clin. 2008. 26:529–540. viii

13. Romero LS, Goltz RW, Nagi C, Shin SS, Ho AD. Subcutaneous T-cell lymphoma with associated hemophagocytic syndrome and terminal leukemic transformation. J Am Acad Dermatol. 1996. 34:904–910.

14. Magro CM, Crowson AN, Kovatich AJ, Burns F. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001. 28:235–247.

15. Reimer P, Rüdiger T, Müller J, Rose C, Wilhelm M, Weissinger F. Subcutaneous panniculitis-like T-cell lymphoma during pregnancy with successful autologous stem cell transplantation. Ann Hematol. 2003. 82:305–309.

16. Mukai HY, Okoshi Y, Shimizu S, Katsura Y, Takei N, Hasegawa Y, et al. Successful treatment of a patient with subcutaneous panniculitis-like T-cell lymphoma with high-dose chemotherapy and total body irradiation. Eur J Haematol. 2003. 70:413–416.

17. Weenig RH, Ng CS, Perniciaro C. Subcutaneous panniculitis-like T-cell lymphoma: an elusive case presenting as lipomembranous panniculitis and a review of 72 cases in the literature. Am J Dermatopathol. 2001. 23:206–215.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download