Abstract
A patient had undergone left pneumonectomy for lung cancer and had an increased risk of fatal complications such as pneumonia, including acute respiratory distress syndrome (ARDS). The treatment effects of veno-venous extracorporeal membrane oxygenation (VV-ECMO) for ARDS of postpneumonectomy patient are uncertain. A 74-year-old man with one lung experienced aspiration pneumonia while swallowing pills after the operation, and his condition progressed to ARDS within a day. He was successfully treated with VV-ECMO support and intensive care unit care.
Acute lung injury and acute respiratory distress syndrome (ARDS) from aspiration pneumonia can lead to severe hypoxemia, disability in pulmonary function, and increased mortality rates from (increase from 30% to 60%).[1-3] Veno-venous extracorporeal membrane oxygenation (VV-ECMO) can be used to treat acute severe respiratory failure that does not respond to mechanical ventilator support. However, ARDS in a patient who has undergone pneumonectomy for lung cancer may not resolve with VV-ECMO. The risk of mortality and morbidity from ARDS is greater in pneumonectomy patients than in those who undergo smaller pulmonary resections or no resections.[4] We report the effectiveness of ECMO in a patient with ARDS following aspiration pneumonia after left pneumonectomy in a patient in the intensive care unit (ICU).
A 74-year-old man had undergone left pneumonectomy for lung cancer 35 years ago, partial laryngectomy for laryngeal cancer 1 year ago, and had been receiving medication for depression because of right lower quadrant abdominal pain for about 1 day. He visited the emergency room and was diagnosed with acute appendicitis. Therefore, he underwent an appendectomy and was discharged on postoperative day 3. Three days later, he visited the emergency room because of operative site pain, fever, nausea, and vomiting. He was diagnosed with postoperative wound infection and postoperative ileus, for which he was admitted. He underwent an incision and drainage for 15 min in the operating room under local anesthesia.
The patient’s oral intake was restricted, and nasogastric tube drainage was maintained because of the ileus. However, he was eager to take only sedatives with sips of water before sleeping because of insomnia. That night, the pills stuck in his throat. Hence, he drank water, following which he complained of cough and severe dyspnea. Chest radiography showed diffuse opacity in the right lung (Fig. 1A), and oxygen saturation (SaO2) level dropped to 82%. He was transferred to the ICU immediately. Subsequently, endotracheal intubation was performed and a ventilator with volume control mode was used to maintain the inspired oxygen fraction (FiO2) at 100%. The tidal volume was 300 mL, positive end-expiratory pressure (PEEP) was 7 cmH2O and respiratory rate (RR) was 30. After initiation of mechanical ventilation, the SaO2 level of arterial blood gas increased to 93%. On hospital day (HD) 2, the patient was more stable than on the previous day. FiO2 dropped to 75% and SaO2 level was maintained at over 93%.
However, at dawn on HD 3, SaO2 dropped to 90% on the monitor, although FiO2 was 100%, PEEP was 15 cmH2O, and tidal volume was 250 mL; RR remained 25. Blood pressure reached only 125/77 mmHg even with high-dose vasopressors (the infusion rate of norepinephrine increased to 8 μg/min; these of dobutamine and dopamine were 10 μg/kg/min and 2.0 μg/kg/min, respectively). Pulse rate also increased to 141 beats/min. Chest anteroposterior radiograph showed aggravation of diffuse opacity and infiltration in the whole right lung (Fig. 1B), and the results of the arterial blood gas analysis (ABGA) showed hypoxemia with severe hypercapnic respiratory acidosis (Table 1). The doctor in charge judged that mechanical ventilation was not sufficient to ventilate oxygen and carbon dioxide. The cardiac surgeon agreed to use VV-ECMO. While preparing for VV-ECMO, epinephrine infusion was started to maintain blood pressure, and a muscle relaxant was administered. The infusion rate of epinephrine was 1 μg/min and that of the muscle relaxant (vecuronium) was 4 mg/hr. Continuous endotracheal suction was performed until ECMO (Maquet Medical Systems USA, Wayne, NJ, USA) was initiated. A 17 French Biomedicus cannula (Medtronic Incorporated, Minneapolis, MN, USA) was inserted in the right femoral vein and was used as the return. A 21 French Biomedicus cannula was inserted in the left femoral vein and used as the drain. Moreover, extracorporeal circulation was started 30 min after the catheters had been placed. Subsequently, RR and pulse rate were stabilized below 20/min and 100/min respectively just 30 minutes after extracorporeal circulation began. SaO2 was maintained at over 95%. The ventilator settings were changed to FiO2 of 0.5, 250 mL tidal volume, 12/min RR, and 5 cmH2O PEEP. The initial oxygen fraction, sweep gas rate, and average flow rate of VV-ECMO were 100%, 4 L/min, and 3,600 mL/min, respectively. The ABGA results are shown in Table 1. The patient’s mean arterial pressure was maintained over at 60 mmHg, and we tapered the dose of epinephrine and norepinephrine. Hourly urine output was over 100 cm3. Antibiotics (vancomycin 1 g b.i.d and metronidazole 500 mg t.i.d) were administered.
The ABGA results in the morning of HD 4 (ECMO day 2) are shown in Table 1. Right upper lobe haziness that had been observed on the chest radiograph had improved. However, lower diffuse consolidation had not improved (Fig. 1C). Vital signs were also stable until the ECMO weaning day. On HD 8, 6 days after the application of ECMO, the patient was weaned from extracorporeal circulation at 10 am, and could tolerate a conventional ventilator alone (pressure control mode of 45% FiO2, 350 mL tidal volume, RR of 13/min, 7 cmH2O PEEP) (Fig. 1D). There were no signs of organ failure or complications following ECMO. However, RR increased up to 30/min, ABGA indicated hypercapnia and respiratory acidosis over time. Then, the ventilator setting was modulated (volume control mode of 45% FiO2, 290 mL tidal volume, RR of 15/min, 7cmH2O PEEP), and the partial pressure of carbon dioxide (PaCO2) was decreased to around 48 mmHg. Follow-up chest radiography was performed. (Fig. 1E). Leukocytosis was 22,100/mm3 (84.6% neutrophil segment) and C-reactive protein (CRP) level was 83 mg/L.
On HD 9, Pseudomonas aeruginosa was isolated from his bronchial aspiration. Ceftazidime 2 g t.i.d and clindamycin 600 mg t.i.d were injected. On HD 14, the patient had a fever with a body temperature of more than 38°C, and pneumonic infiltration in the right lower lung field was observed on radiography (Fig. 1F). Multi-drug resistant Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus were isolated from his bronchial aspiration. Meropenem 500 mg t.i.d, ampicillin/sulbactam 1.5 g q.i.d, and vancomycin 1 g b.i.d were administered. The white blood cell count was 16,200/μL (84.3% neutrophil segment) and CRP level was 108 mg/L. the appendectomy site was cleaned and had no signs of infection. After the antibiotics were changed, fever subsided.
On HD 15, the patient’s mental status was clear and he was responsive to commands, SaO2 was maintained over 95%, and weaning and extubation were performed. However, the next day the patient complained of breathlessness and SaO2 dropped to 80%; re-intubation was performed and ventilator care was restarted. Subsequently, weaning was attempted consistently.
On HD 21, percutaneous drainage was carried out to relieve pleural effusion of the right lung field. On HD 27, since weaning failed, tracheostomy was performed. Vancomycin was used for 2 weeks and meropenem and ampicillin/sulbactam were used to treat hospital-acquired pneumonia for 3 weeks. ICU care was continued for 2 months because of high pCO2 and increased RR.
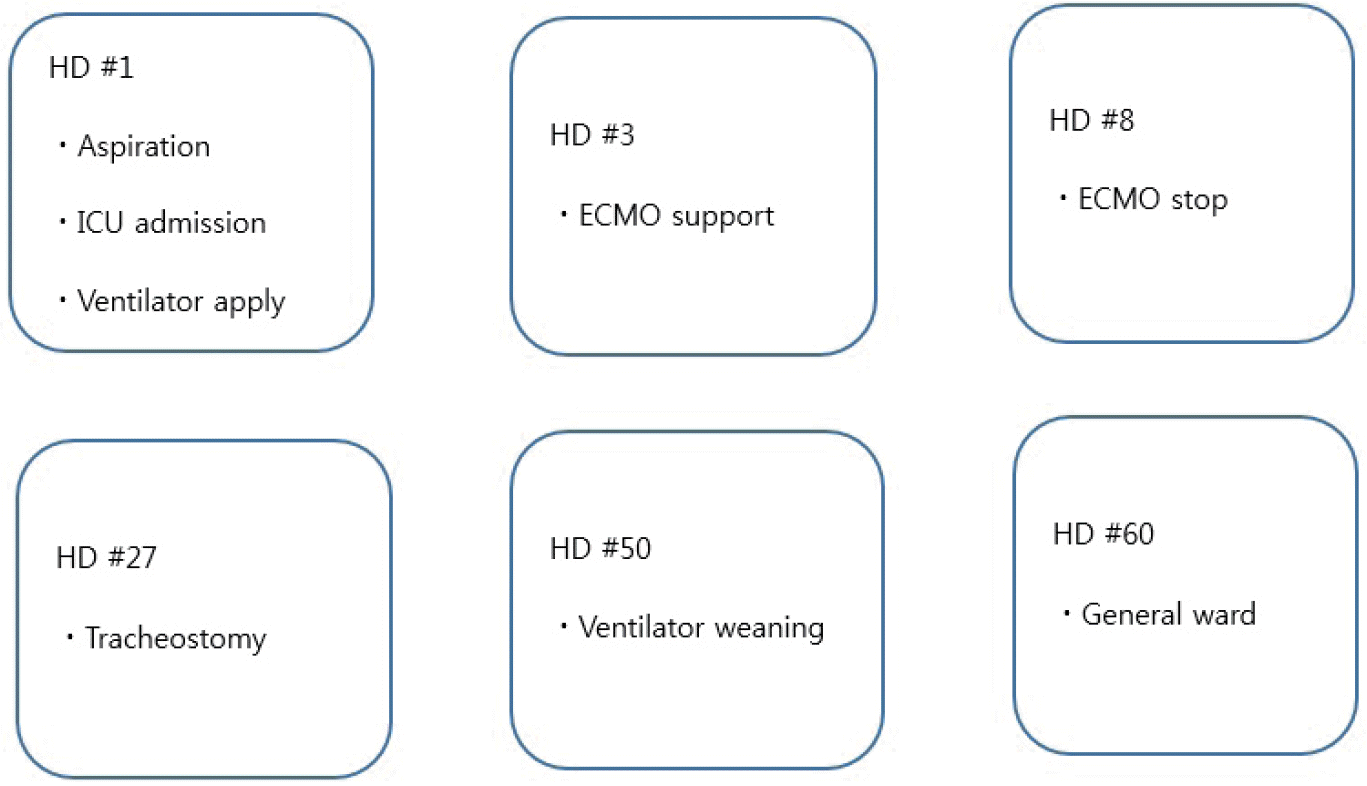
From HD 50, ventilator weaning with pressure support mode was started using the pressure control mode. The patient’s condition was comparatively tolerable, but PaCO2 reached 50 mmHg. On HD 56, the ventilator was removed and a T-piece was connected with FiO2 of 35%. The vital signs were stable. The ABGA results are shown in Table 1. Although PaCO2 was high, the patient did not complain of dyspnea. The white blood cell count was 9,800/μL (75.1% neutrophil segment) and CRP level was 20.98 mg/L. On HD 60, the patient was transferred to the general ward. The brief course of the patient’s condition is shown in Fig. 2.
If cases of severe hypoxemia and hypercapnic acidemia do not improve with conventional mechanical ventilator support, ECMO can be used for life support.[3] VV-ECMO is recommended for isolated lung failure without cardiac failure. This technique, which allows optimal gas exchange, has gained consensus as a supportive treatment when conventional mechanical ventilation fails.[5] Nevertheless, VV-ECMO is a procedure associated with high costs and resource utilization.[6] VV-ECMO use should be carefully determined for high-risk patients.
In this case, the patient was >70 years old and had undergone a left pneumonectomy for lung cancer and partial laryngectomy for laryngeal cancer. This patient had many risk factors for mortality and morbidity that may not be an indication of ECMO therapy.
There have been cases of VV-ECMO use in ARDS postpneumonectomy. One was a case of multifactorial ARDS (pneumonia, polytransfusion, and fluid overload) after a right pneumonectomy because of blunt chest trauma. The other was a case of left pneumonectomy because of cystic fibrosis. Both cases involved young patients who were 25 and 31 -years -old, respectively.[7,8]
Pneumonectomy results in pulmonary function deficits and decreases exercise capacity.[9,10] Forced vital capacity, forced expiratory volume in 1 s, and diffusion capacity for carbon monoxide are all reduced postsurgery, with a more significant decline.[9,10] Most reports showed that postpneumonectomy patients have diminished exercise tolerance due to mechanical respiratory limitation, low ventilator capacity, reduced breathing reserve, and dyspnea without significant differences between right or left lung removal.[11-15] Pneumonectomy is associated with several anatomical changes within the thoracic cavity that may reduce maximal cardiac output and attenuate heart function.[10] Most patients can maintain nearnormal life in activities of daily living, but the conditions such as pneumonia or ARDS are fatal postpneumonectomy or the abovementioned reasons.[16]
Aspiration pneumonia is treated with antibiotics. ECMO is just a supportive unit that can be used to control lung functions, even in pneumonectomy patients. Hospitalacquired infections are still most common among patients who had been recently hospitalized and infections include those by gram-negative bacteria such as Pseudomonas aeruginosa and Klebsiella pneumoniae as well as methicillinresistant Staphylococcus aureus. Piperacillin/tazobactam or imipenem/cilastatin plus vancomycin would be appropriate for treatment of such infections.[17] Further, appropriate antibiotic regimens must be chosen according to sputum culture results. In this case, we prescribed vancomycin and metronidazole at first. However, there were no grampositive cocci on culture, of a single on the sputum stain. The regimens were inappropriate, and pneumonia might be aggravated. The antibiotics were changed to ceftriaxone and clindamycin to treat Pseudomonas in the sputum after the infecting bacteria were identified. After 2 weeks, the patient had fever and the haziness of the right lung was enhanced. The medication was changed to meropenem and ampicillin/sulbactam to treat multi-drug resistant Acinetobacter baumannii.
In conclusion, the medical team could consider ECMO to treat aspiration pneumonia in postpneumonectomy patients or other high-risk patients. Once aspiration pneumonia occurs, the respiratory system can develop pneumonitis, pneumonia, and acute lung injury, including ARDS. If severe hypoxemia and hypercapnia occurs and optimal gas exchange cannot achieved by conventional mechanical ventilation, extracorporeal life support can have survival benefits even in postpneumonectomy.
References
1. Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999; 83:453–60.

2. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993; 78:56–62.

3. Kim N, Kim KH, Kim JM, Choi SY, Na S. Early extracorporeal membrane oxygenation for massive aspiration during anesthesia induction. Korean J Crit Care Med. 2015; 30:109–14.

4. Harpole DH, Liptay MJ, DeCamp MM Jr., Mentzer SJ, Swanson SJ, Sugarbaker DJ. Prospective analysis of pneumonectomy: risk factors for major morbidity and cardiac dysrhythmias. Ann Thorac Surg. 1996; 61:977–82.

5. Diaz JV, Brower R, Calfee CS, Matthay MA. Therapeutic strategies for severe acute lung injury. Crit Care Med. 2010; 38:1644–50.

6. Enger T, Philipp A, Videm V, Lubnow M, Wahba A, Fischer M, et al. Prediction of mortality in adult patients with severe acute lung failure receiving venovenous extracorporeal membrane oxygenation: a prospective observational study. Crit Care. 2014; 18:R67.

7. Saueressig MG, Schwarz P, Schlatter R, Moreschi AH, Wender OC, Macedo-Neto AV. Extracorporeal membrane oxygenation for postpneumonectomy ARDS. J Bras Pneumol. 2014; 40:203–6.

8. Martucci G, Panarello G, Bertani A, Occhipinti G, Pintaudi S, Arcadipane A. Veno-venous ECMO in ARDS after post-traumatic pneumonectomy. Intensive Care Med. 2013; 39:2235–6.

9. Powell ES, Pearce AC, Cook D, Davies P, Bishay E, Bowler GM, et al. UK pneumonectomy outcome study (UKPOS): a prospective observational study of pneumonectomy outcome. J Cardiothorac Surg. 2009; 4:41.

10. Deslauriers J, Ugalde P, Miro S, Deslauriers DR, Ferland S, Bergeron S, et al. Long-term physiological consequences of pneumonectomy. Semin Thorac Cardiovasc Surg. 2011; 23:196–202.

11. Smulders SA, Smeenk FW, Janssen-Heijnen ML, Postmus PE. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest. 2004; 125:1735–41.
12. Deslauriers J, Ugalde P, Miro S, Ferland S, Bergeron S, Lacasse Y, et al. Adjustments in cardiorespiratory function after pneumonectomy: results of the pneumonectomy project. J Thorac Cardiovasc Surg. 2011; 141:7–15.

13. Nugent AM, Steele IC, Carragher AM, McManus K, McGuigan JA, Gibbons JR, et al. Effect of thoracotomy and lung resection on exercise capacity in patients with lung cancer. Thorax. 1999; 54:334–8.

14. Larsen KR, Svendsen UG, Milman N, Brenøe J, Petersen BN. Cardiopulmonary function at rest and during exercise after resection for bronchial carcinoma. Ann Thorac Surg. 1997; 64:960–4.
15. Pelletier C, Lapointe L, LeBlanc P. Effects of lung resection on pulmonary function and exercise capacity. Thorax. 1990; 45:497–502.

16. Vainshelboim B, Fox BD, Saute M, Sagie A, Yehoshua L, Fuks L, et al. Limitations in exercise and functional capacity in long-term postpneumonectomy patients. J Cardiopulm Rehabil Prev. 2015; 35:56–64.

17. Anand S. Aspiration pneumonitis and pneumonia. 2015. [2015 April]. Available from http://emedicine.medscape.com/article/296198-overview.

Fig. 1.
Serial chest radiographs. (A) Hospital day (HD) number #1, immediately after aspirating water. (B) HD number #3, in intensive care unit. (C) On day 2 of extracorporeal membrane oxygenation (ECMO). (D) Improved consolidation in right lung on day 6 of ECMO. (E) 1 hour after weaning from ECMO. (F) HD number #14, pneumonic infiltration in right lower ling field.
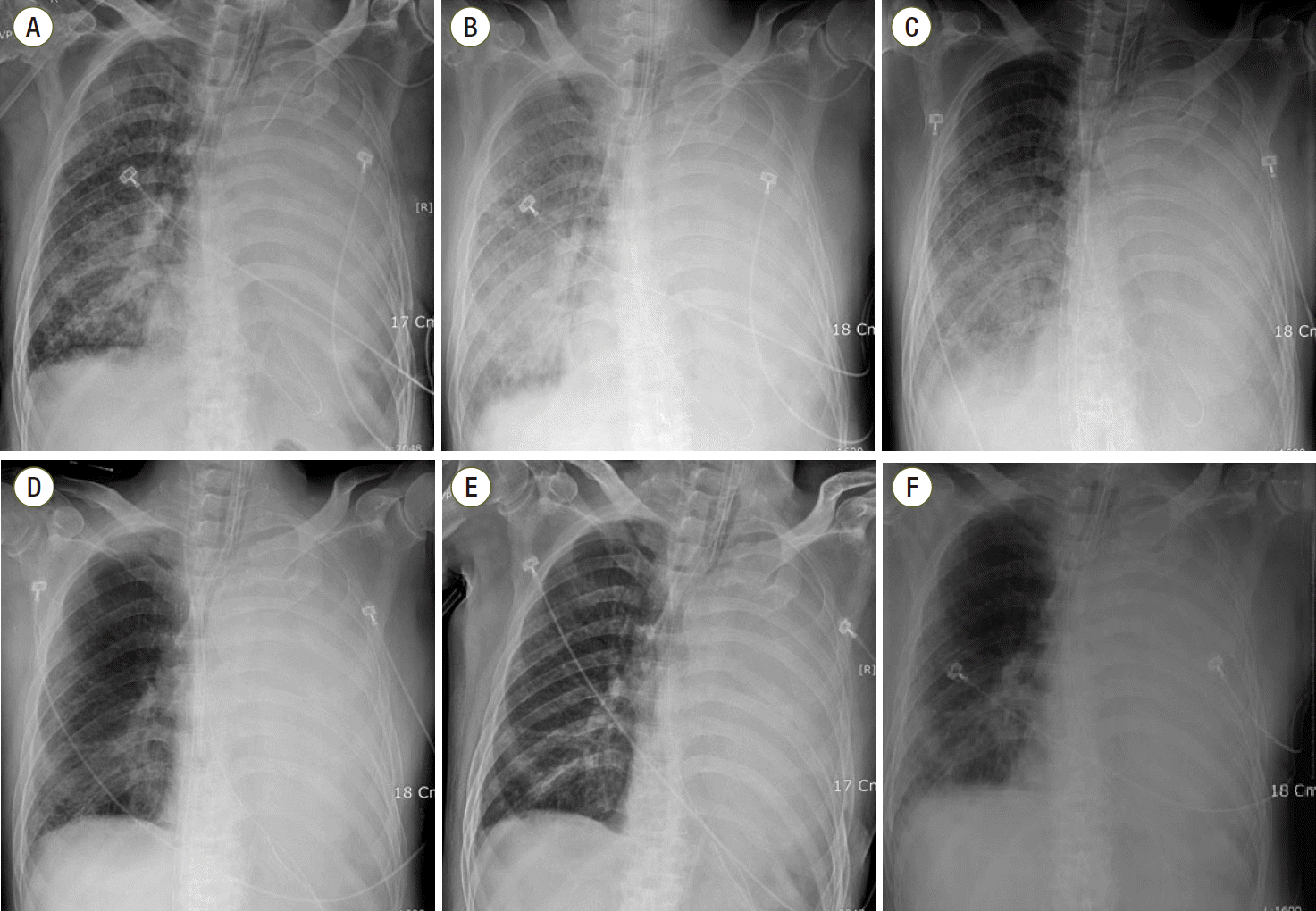
Fig. 2.
Brief course of the patient. HD: hospital day; ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download