Abstract
Opioid-induced chest wall rigidity is an uncommon complication of opioids. Because of this, it is often difficult to make a differential diagnosis in a mechanically ventilated patient who experiences increased airway pressure and difficulty with ventilation. A 76-year-old female patient was admitted to the intensive care unit (ICU) after surgery for periprosthetic fracture of the femur neck. On completion of the surgery, airway pressure was increased, and oxygen saturation fell below 95% after a bolus dose of fentanyl. After ICU admission, the same event recurred. Manual ventilation was immediately started, and a muscle relaxant relieved the symptoms. There was no sign or symptom suggesting airway obstruction or asthma on physical examination. Early recognition and treatment should be made in a mechanically ventilated patient experiencing increased airway pressure in order to prevent further deterioration.
Opioid-induced chest wall rigidity is characterized by increased muscle tone particularly in the thoracic and abdominal muscles after administration of opioids. In an intubated patient with abruptly elevated airway pressure, acute asthmatic attack and bronchospasm are relatively common, however, opioid-induced chest wall rigidity also should be considered in a patient with neither wheezing nor chest wall movement. We report a case of recurrent opioid-induced chest wall rigidity induced with low dose fentanyl, which was treated successfully with muscle relaxant.
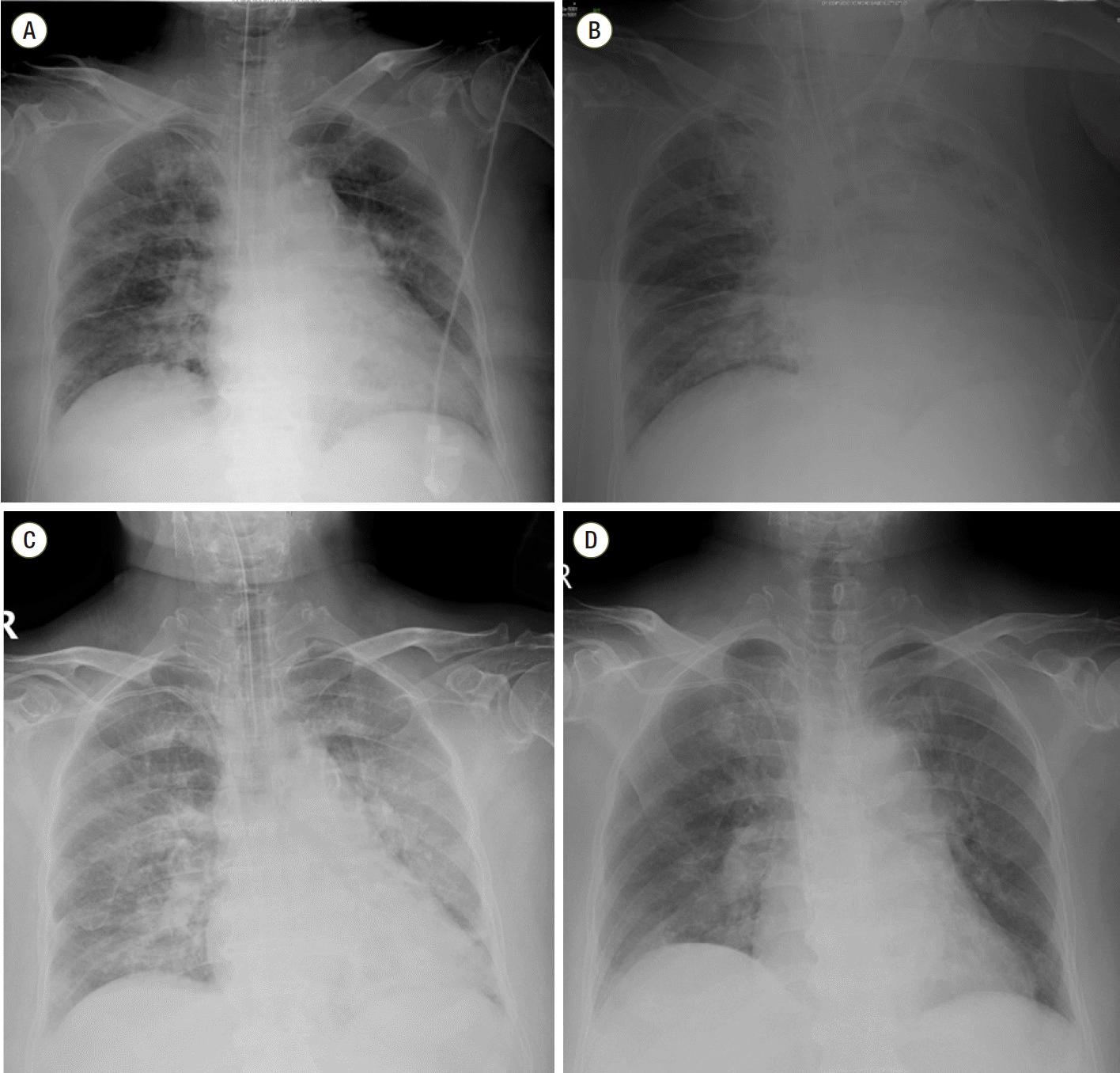
A 76-year-old female patient was transferred to the intensive care unit (ICU) after surgery for periprosthetic fracture of femur neck. She had previous history of hypertension, diabetes mellitus, coronary artery occlusive disease, chronic kidney disease and asthma. And there was no significant adverse event during the previous anesthesia for the surgery of femur neck fracture a week before. Preoperative chest x-ray showed focal bronchiectasis at left lower lung field and there was no definite abnormal lung consolidation or collapse. Anesthesia was induced with propofol 2 mg/kg, sevoflurane, and remifentanil 0.2 μg/kg, and rocuronium 0.6 mg/kg was used to facilitate endotracheal intubation. After induction of anesthesia, vital signs were blood pressure 140/53 mmHg, heart rate 84 beats/min and pulse oximetry 99% with FiO2 50%. Anesthesia was maintained with 1.0 minimum alveolar concentration of sevoflurane and remifentanil 0.05-2 μg/kg/min. Airway pressure was about 19-22 cmH2O during the anesthetic time. After completion of the surgery and before extubation, intravenous bolus of fentanyl 50 mcg was administered, and then airway pressure was abruptly elevated to 36 mmHg and oxygen saturation fell below 95%. There was no wheezing or stridor at this point. Immediate chest x-ray showed left pleural effusion which could not explain desaturation event (Fig. 1). Elevated airway pressure was maintained for 10 minutes and arterial blood gas analysis performed at that time did not show significant hypoxemia or hypercarbia (pH, 7.380; PaCO2 38.8 mmHg; PaO2 173.6 mmHg; and oxygen saturation 99.6%; FiO2 of 50%). And there was no delayed rise of the end-tidal CO2 (EtCO2) on capnograph which can suggest airway obstruction. EtCO2 was 36-38 mmHg. At this point, it was decided to transfer the patient not to the ward but to the ICU in intubated status and 25 mg of atracurium (Atra®, Hana, Seoul, Korea) and 3 mg of midazolam were administered. On admission to ICU, FiO2 of 50%, positive end expiratory pressure (PEEP) of 5 cmH2O was applied and peak inspiratory pressure was 32 mmHg. And hypoxemia was noticed again by arterial blood gas analysis (ABGA) as PaO2 55.2 mmHg; oxygen saturation 86.5%. Thus, FiO2 was increased to 60% and PEEP was increased to 8 cmH2O. The patient developed hypertension to 200/96 mmHg at this time, perdipine infusion was started. After ICU arrival, intravenous patient-controlled analgesia (PCA) consisting of fentanyl 20 μg/kg and 0.9% saline in a total volume of 100 mL (basal rate 2 mL/hr, bolus dose 0.5 mL, lockout time 15 minutes) was connected to the patient. One hour after ICU admission, oxygen saturation fell to 95%, tidal volume was not checked and airway pressure was elevated to 40 cmH2O on the ventilator. After adjustment of ventilator setting as FiO2 70% and PEEP 5 cmH2O, ABGA showed optimal oxygenation; pH 7.393; pCO2 35.6; pO2 321.8; and oxygen saturation 99.9%. Auscultation revealed no evidence of wheezing or stridor at this point. Fiberoptic bronchoscopy (FOB) was performed immediately and there was no endobronchial lesion. And there was generally whitish secretion in the bronchus. There was no eosinophilia on complete blood count with differential white blood cell analysis. Eosinophil percentage was 1% and eosinophil count was 65/μL. Three hours after ICU arrival, tidal volume was not checked again and oxygen saturation fell to 60%. FiO2 was increased to 100% and manual bag-valve-mask ventilation was initiated. Intravenous PCA was disconnected. At that time, minimal chest wall movement was observed and high airway pressure was needed to deliver tidal volume. Opioid-induced chest wall rigidity was suspected at this time, and vecuronium bromide (Norcuron®, MSD, Seoul, Korea) 8 mg was injected, immediately. After relaxation was achieved, peak airway pressure was decreased to 19 mmHg and ventilation was feasible smoothly. Thus, cisatracurium (Nimbex®, GSK, Seoul, Korea) infusion was started. Transthoracic echocardiography was performed for evaluation of heart failure and there were no specific findings. The next day, the patient was extubated successfully after spontaneous breathing trial.
Increase of airway pressure is common in ventilated patients and early recognition and treatment is essential to prevent further desaturation and hypoxemia. In a patient with increased airway pressure with difficult ventilation, acute asthmatic attack should be considered. Other possible considerations include upper airway obstruction such as vocal cord dysfunction and laryngeal malignancy, bronchospasm, and opioid-induced chest wall rigidity. But in this case, the patient was intubated status and we could easily exclude the possibility of upper airway obstruction by passing the suction catheter through the tube. Physical findings of the absence of wheezing or chest wall movement precludes asthma from possible diagnosis, and opioid-induced chest wall rigidity is more feasible in this clinical findings.
Opioid-induced chest wall rigidity is characterized by increased muscle tone particularly in the thoracic and abdominal muscles after administration of opioids. Opioid-induced chest wall rigidity usually has been reported after high-dose fentanyl (17 μg/kg) administration.[1] But several previous studies reported that even a small repeated bolus dose of fentanyl (50 μg once, total dose of 200-250 μg) may also cause chest wall rigidity.[2-4] Furthermore, this phenomenon often reported to appear several hours after administration of opioids.[5,6] The exact mechanism responsible for this phenomenon is not known. But several experimental studies suggested possible mechanisms. Vankova et al.[7] demonstrated that opioid-induced muscular rigidity is primarily due the activation of central mu receptors. Another study reported that central dopaminergic pathway might be at least partially responsible for the opioid-induced chest wall rigidity.[8] Others suggested that the coerulospinal noradrenergic pathway may be directly involved in the elicitation of the muscular rigidity by fentanyl, possibly via alph 1-adrenoreceptors in the spinal cord.[9,10] The mechanism of this phenomenon remains unclear, but several risk factors have been suggested. Chest wall rigidity after fentanyl administration usually associated with rapid injection, large doses, and extremes of age (e.g., newborns, elderly patients).[2,11] In a previous report, average dose of 19 ± 1.9 μg/kg of fentanyl was administered at the rate of 200 μg/min and truncal rigidity occurred in 20 of 21 patients.[12] And Jaffe and Ramsey reported that the incidence of severe rigidity was 88 % with more rapid infusion of fentanyl (1 mg/min).[13] In this case, small bolus dose of fentanyl (50 μg) was administered once. However, there was no significant adverse event such as chest wall rigidity during the previous general anesthesia a week before. But in a previous surgery, remifentanil continuous infusion was used and the bolus dose of fentanyl was not used. Thus, we suspect that rapid injection of small bolus dose of fentanyl could be a cause of chest wall rigidity. And the recurrent episodes of desaturation lasted to three hours after surgery. Opioid-induced chest wall rigidity is well known phenomenon. But to our knowledge, recurrent episodes of chest wall rigidity have not been reported previously.
Patients experienced opioid-induced chest wall rigidity was successfully treated with naloxone in previous studies. [2,11,14] But in this case, we administered muscle relaxant. Owing to the atypical feature in this patient, we should consider the possibility of other probable causes. Considering the urgency of hypoxemia events, immediate response to the treatment should be secured. From this point of view, we preferred immediate response of muscle relaxant rather waiting slow onset of opioid antagonist. But Jaffe and Ramsey already suggested the use of muscle relaxant as pretreatment to prevent opioid-induced chest wall rigidity. [13] And in another case report, authors successfully treated opioid-induced muscular rigidity by administrating suxamethonium 100 mg.[3]
Because the episodes were recurrent, we also suspected acute asthmatic attack. Asthma is a disease characterized by airway inflammation and bronchial hyperresponsiveness. As previously described, key signs of severe asthma include tachycardia, tachypnea, anxiety, diaphoresis, and inability to speak.[15] In a mechanically ventilated patient, the symptoms of acute asthmatic attack may represent as elevated airway pressure and difficult ventilation.
In our case, it is difficult to make differential diagnosis because she was already intubated and sedated. Generally, the symptoms and signs of the patient are important to make a diagnosis. In an intubated patient, capnogram and auscultation can be clues. A patient with obstructive lung disease such as bronchospasm and acute asthmatic attack can be suspected by capnogram. A capnogram will show delayed rise of end tidal CO2 in obstructive patients. Auscultation also is helpful. There was no wheezing or stridor in this patient. Thus, we thought that the possibility of asthmatic attack is low.
In addition, the patient became hypertensive and this finding is in accordance with the previous case report. [3] In agreement with these case reports, Neidhart et al.[16] revealed that arterial pressure showed statistically significant decrease in patients without muscle rigidity at the end of the fentanyl infusion. On the other hand, there were no significant changes of mean arterial pressure in patients with muscle rigidity. The authors suggested the mechanism of this phenomenon in two ways: the decrease in sympathetic tone caused by induction of anesthesia could counteract the increase in arterial blood pressure caused by increased muscle tension.[16] Thus, normotension or hypertension can be another clue for diagnosis in patients suspecting opioid-induced chest wall rigidity.
Bronchial lavage was successfully used to treat severe status asthmaticus by removing bronchial plugs.[17] In a previous report, authors suggested that increasing airway obstruction was due to plugging of bronchi with inspissated mucus which may form casts of the bronchi several centimeters in length. And they thought that removal of this mucus might lead to clinical improvement.[18] In current case, FOB was done and there was generally whitish secretion in the bronchus. Although this patient was not such a case of severe status asthmaticus, bronchial lavage for differential diagnosis might be helpful to find out the cause of obstruction. Despite low diagnostic value of bronchial lavage, it is worth to try in that it can serve as both diagnostic and therapeutic in a patient with severe obstructive disease.
In summary, we report a case about a patient with recurrent opioid-induced chest wall rigidity induced by low dose fentanyl who treated successfully with muscle relaxant. Early recognition and treatment should be made in a mechanically ventilated patient with elevated airway pressure.
References
3. Dimitriou V, Zogogiannis I, Liotiri D, Wambi F, Tawfeeq N, Koumi A, et al. Impossible mask ventilation after an unusually low dose fentanyl-induced muscle rigidity in a patient with essential tremor: a case report and review of the literature. Middle East J Anaesthesiol. 2014; 22:619–22.
4. Rim SK, Kim JI, Son YB, Lee JH. Muscular rigidity and pulmonary edema following administration of low dose fentanyl: a case report. Korean J Crit Care Med. 2012; 27:197–201.
5. Klausner JM, Caspi J, Lelcuk S, Khazam A, Marin G, Hechtman HB, et al. Delayed muscular rigidity and respiratory depression following fentanyl anesthesia. Arch Surg. 1988; 123:66–7.

6. Kang BJ, Kim SH. Opioid-induced muscle rigidity with a delayed manifestation misunderstood as a tension pneumothorax: a case report. Korean J Pain. 2008; 21:66–70.

7. Vankova ME, Weinger MB, Chen DY, Bronson JB, Motis V, Koob GF. Role of central mu, delta-1, and kappa-1 opioid receptors in opioid-induced muscle rigidity in the rat. Anesthesiology. 1996; 85:574–83.
8. Soares JH, Brosnan RJ, Smith A, Mayhew PD. Rabbit model of chest wall rigidity induced by fentanyl and the effects of apomorphine. Respir Physiol Neurobiol. 2014; 202:50–2.

9. Lui PW, Lee TY, Chan SH. Involvement of coerulospinal noradrenergic pathway in fentanyl-induced muscular rigidity in rats. Neurosci Lett. 1990; 108:183–8.

10. Lui PW, Lee TY, Chan SH. Involvement of locus coeruleus and noradrenergic neurotransmission in fentanyl-induced muscular rigidity in the rat. Neurosci Lett. 1989; 96:114–9.

11. Viscomi CM, Bailey PL. Opioid-induced rigidity after intravenous fentanyl. Obstet Gynecol. 1997; 89(5 Pt 2):822–4.

12. Comstock MK, Carter JG, Moyers JR, Stevens WC. Rigidity and hypercarbia associated with high dose fentanyl induction of anesthesia. Anesth Analg. 1981; 60:362–3.

13. Jaffe TB, Ramsey FM. Attenuation of fentanyl-induced truncal rigidity. Anesthesiology. 1983; 58:562–4.

14. Ackerman WE, Phero JC, Theodore GT. Ineffective ventilation during conscious sedation due to chest wall rigidity after intravenous midazolam and fentanyl. Anesth Prog. 1990; 37:46–8.
15. Mannam P, Siegel MD. Analytic review: management of life-threatening asthma in adults. J Intensive Care Med. 2010; 25:3–15.

Fig. 1.
Perioperative chest radiograph. (A) Preoperative chest radiograph showed focal bronchiectasis at left lower lung field and there was no definite abnormal lung consolidation or collapse. (B) Postoperative chest radiograph showed left pleural effusion (C) Three hours after ICU arrival, the amount of pleural effusion was decreased. (D) On POD#2, there was no definite abnormal lung consolidation or collapse. ICU: intensive care uint.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download