Abstract
A 51-year-old male patient was referred for a sudden out-of-hospital cardiac arrest. Upon arrival, he was conscious and had no chest pain complaints. There was no abnormality in initial electrocardiographic and echocardiographic examinations. However, episodes of recurrent ventricular fibrillation (VF) were documented on rhythm monitoring. Each VF episode was triggered by an isolated monomorphic ventricular premature complex (VPC). Suspecting idiopathic VF, emergency radiofrequency catheter ablation was planned for the VPCs. However, when coronary angiography was performed to exclude silent ischemia, the results showed a total occlusion of the right coronary artery posterolateral branch, which is thought to supply the left ventricular inferior and septal wall. After successful reperfusion, VF episodes and the triggering VPCs disappeared. We are documenting this case to emphasize the potential for silent myocardial infarction to cause out-of-hospital sudden cardiac arrest even in a patient without any symptom or sign of acute coronary syndrome.
Although a variety of diseases can cause sudden cardiac arrest, the most common cause of out-of-hospital sudden cardiac arrest is coronary artery disease including acute myocardial infarction.[1] Current guidelines recommend early invasive coronary angiography for out-of-hospital sudden cardiac arrest survivors with symptoms suggestive of acute coronary syndrome (ACS).[2] However, it is difficult to surmise the possibility of ACS in patients without typical symptoms or objective signs of myocardial ischemia. We report a case of out-of-hospital sudden cardiac arrest with recurrent ventricular fibrillations (VFs) caused by an acute but asymptomatic focal myocardial infarction.
A 51-year-old male patient suddenly collapsed at home. The sudden collapse was witnessed by his wife and chest compressions were initiated immediately. An ambulatory rescue team arrived at the house 8 minutes later and employed an automated external defibrillator. The documented presenting rhythm was a VF, which was successfully terminated by a single automated external defibrillator shock of 150J. The patient was transferred to the hospital emergency room after the return of spontaneous circulation. Upon arrival, the patient was conscious and did not complain chest pain. Vital signs were stable (blood pressure: 90/60 mmHg, heart rate: 72 bpm) and physical examinations revealed nothing remarkable. The patient had a smoking history of 30 pack-years and had been treated for hypertension for over 5 years with amlodipine 5 mg and hydrochlorothiazide 12.5 mg a day. Laboratory investigations were unremarkable except for elevated cardiac troponin-I level (0.831 ng/mL). Initial standard 12-lead ECGs showed atrial fibrillation with occasional ventricular premature complexes (VPCs). There were J-point elevations (0.1–0.2 mV) with early repolarization patterns in limb leads II, III, and aVF. However, there was no typical ST-segment elevation or depression (Fig. 1). In addition, emergency echocardiographic examination showed normal left ventricular wall motion with an ejection fraction of 62%.
The patient was admitted to a coronary care unit for continuous rhythm monitoring. In the initial 3 hours after admission, isolated monomorphic VPCs appeared frequently (5–6 VPCs per minute) on the monitor and episodes of VF recurred 7 times. Recurrent VFs were successfully terminated by immediate external defibrillations. When the patient became conscious after defibrillation, he again had no chest pain complaints. Repeated electrocardiographic and echocardiographic examinations performed between the VF episodes did not reveal any abnormalities. However, recorded rhythm analysis demonstrated that all 7 episodes of the VFs were initiated by an isolated monomorphic VPC (Fig. 2). The monomorphic VPCs documented on 12-lead electrocardiogram (ECGs) exhibited right bundle branch block configurations in V1, superior axes, and positive QRS deflections in V1–V5. The morphologic characteristics of the QRS complexes of the monomorphic VPCs suggested an inferior or septal wall origin. Because there were no symptoms or signs suggestive of ACS, the initial diagnosis was made as an idiopathic VF triggered by unifocal VPCs. Although the initial troponin-I level was elevated, it was regarded as a consequence of prolonged cardiopulmonary resuscitation. After 2 hours from visit, amiodarone 300 mg was administered intravenously to suppress VPC recurrence, which was unsuccessful. Rapid right ventricular pacing at a rate of 100 bpm using a transvenous temporary pacemaker also failed to suppress VPC recurrence. Cardiac troponin-I level after 3 hours from initial visit showed minimal increase (0.927 ng/mL), which was also regarded as a result of VF and defibrillation. An emergency electrophysiological study was planned with radiofrequency catheter ablation for the VPCs. However, prior to performing radiofrequency ablation, we conducted a diagnostic coronary angiography to exclude the possibility of silent myocardial ischemia after 4 hours from the visit. Left coronary angiography showed no significant luminal stenosis. Right coronary angiography results showed a total occlusion of the small posterolateral branch, which supplied the left ventricular posterior and posterolateral wall near the base (Fig. 3A and 3C). Although the location of the angiographically-confirmed culprit lesion did not correspond to the postulated VPC origin perfectly, it was not far from the origin supposed from the morphologic characteristics of the QRS complexes of the monomorphic VPCs. Balloon angioplasty was performed for the acute total occlusive lesion immediately and Thrombolysis in Myocardial Infarction grade 3 flow was restored at the end of the intervention (Fig. 3B and 3D). After successful reperfusion, the frequency of monomorphic VPCs decreased remarkably within 5 minutes and a few remaining VPCs did not result in the induction of VF. The monomorphic VPCs disappeared nearly completely 10 minutes later. Radiofrequency ablation was not necessary and we decided to observe the patient while maintaining dual antiplatelet agents and heparin with continuous ECG monitoring. Follow-up ECGs after coronary artery intervention showed no significant ST-T wave changes. However, cardiac troponin-I was increased as 3.245 ng/mL. The patient was discharged 7 days later, after confirmation of the complete disappearance of the monomorphic VPCs with continuous ECG monitoring. There was no evidence of VPC recurrence on repeated Holter monitoring during the follow-up duration over 1 year.
ACS is the most common cause of out-of-hospital sudden cardiac arrest.[1] Current guidelines recommend an early invasive strategy including coronary angiography for out-of-hospital cardiac arrest survivors with symptoms or signs suggestive of ACS.[2] However, in this case, there was no chest pain, significant ST-T wave changes on ECG, or wall motion abnormalities on echocardiography. The elevated troponin-I level was not regarded as a sign of acute myocardial infarction due to it being a common finding in patients resuscitated from cardiac arrest by any cause. In the present case, VF following VPCs in a patient without significant sign or symptom of ACS were treated with reperfusion. Although VPCs are well known risk factors for the development of fatal ventricular arrhythmia in patients with or without ischemic heart diseases,[3] the monomorphic VPCs are frequently regarded as insignificant findings. We did not think the VPCs significant either. Even when the initiation of VF by unifocal VPCs was documented, it was diagnosed as an idiopathic VF triggered by unifocal VPCs in patients without structural or ischemic heart disease. It is well known that idiopathic VFs can be triggered by isolated or a series of unifocal VPCs, and treated by radiofrequency catheter ablation.[3–5] Unfortunately, the absence of symptoms and signs suggestive of ACS misled incorrect diagnosis and early invasive coronary angiography was delayed. It was thought that focal acute myocardial infarction due to the relatively small posterolateral branch occlusion did not induce significant angina pain, ST-T wave change, or left ventricular wall motion abnormalities. However, the past history of smoking and hypertension suggested the possibility of ACS.
Unless there is another likely cause of cardiac arrest, the possibility of ACS should be kept in mind as the potential cause of sudden cardiac arrest, regardless of the presence of clinical findings suggestive of myocardial ischemia. Current guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death also recommend coronary angiography to evaluate patients resuscitated from out-of-hospital sudden cardiac arrests.[6]
Another insightful finding is the occurrence of VPCs. The location of acute ischemic myocardium can be localized by the QRS complex morphology of the ischemic VPCs. In this case, the postulated VPC origin inferred from the QRS complex morphology of the unifocal VPCs and the angiographically confirmed ischemic myocardial territory was not perfectly matched. However, successful reperfusion of the culprit vessel ceased the unifocal VPCs and episodes of VFs dramatically. Acute myocardial ischemia can result in fatal arrhymia by formation of re-entry circuit. However, it is known that acute myocardial ischemia changes the membrane excitability, action potential duration and recovery of excitability of affected area. These electrical changes are thought to be related with generation of reentry associated VF rather than monomorphic VPCs.[7] Although the VPCs in the present case seem to be associated with focal ischemia from coronary artery occlusion, it is more plausible to explain that the VPCs are associated with increased excitability of non-ischemic myocardium due to increased catecholamine level.[8] However, acute ischemia can also induce the leakage of intracellular potassium into the extracellular matrix, and it is possible to result in abnormal myocardial depolarization.[9]
We report a documented case of out-of-hospital sudden cardiac arrest due to recurrent VF triggered by isolated unifocal VPCs in a patient with acute but silent focal myocardial infarction. Although there were no definitive symptoms or signs suggestive of ACS, the possibility of silent myocardial ischemia should be kept in mind as a potential cause of out-of-hospital sudden cardiac arrest, and a systematic evaluation including coronary angiography should be considered to minimize the risk of misdiagnosis.
References
2). O'Connor RE, Bossaert L, Arntz HR, Brooks SC, Diercks D, Feitosa-Filho G, et al. Part 9: Acute coronary syndromes: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010; 122(16 suppl 2):S422–65.
3). Haissaguerre M, Shoda M, Jais P, Nogami A, Shah DC, Kautzner J, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002; 106:962–7.

4). Tilz RR, Lin T, Makimoto H, Ouyang F. Successful epicardial ablation of electrical storms due to recurrent ventricular fibrillation triggered by premature ventricular contractions. Heart Rhythm. 2014; 11:146–9.

5). Cho YR, Park JS. Radiofrequency catheter ablation for unifocal premature ventricular complexes triggering recurrent ventricular fibrillations in a young man without structural heart disease. Korean Circ J. 2012; 42:575–9.

6). Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e385–484.

7). Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997; 35:256–72.

8). Little RA, Frayn KN, Randall PE, Stoner HB, Morton C, Yates DW, et al. Plasma catecholamines in the acute phase of the response to myocardial infarction. Arch Emerg Med. 1986; 3:20–7.

9). Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ Res. 1997; 80:124–38.
Fig. 1.
A 12-lead ECG findings at initial presentation. Initial 12-lead ECG shows atrial fibrillation with an isolated VPC. There are J-point elevations (0.1–0.2 mV) with early repolarization patterns in limb leads II, III, and aVF. However, there are no typical ST segment elevation or depression seen in patients with acute coronary syndrome. Documented isolated VPCs has a right bundle branch block configurations in V1, superior axes, and positive QRS deflections in V1–V5, suggesting their inferior or septal wall origin near the cardiac base. ECG: electrocardiogram; VPC: ventricular premature complex; VF: ventricular fibrillation.
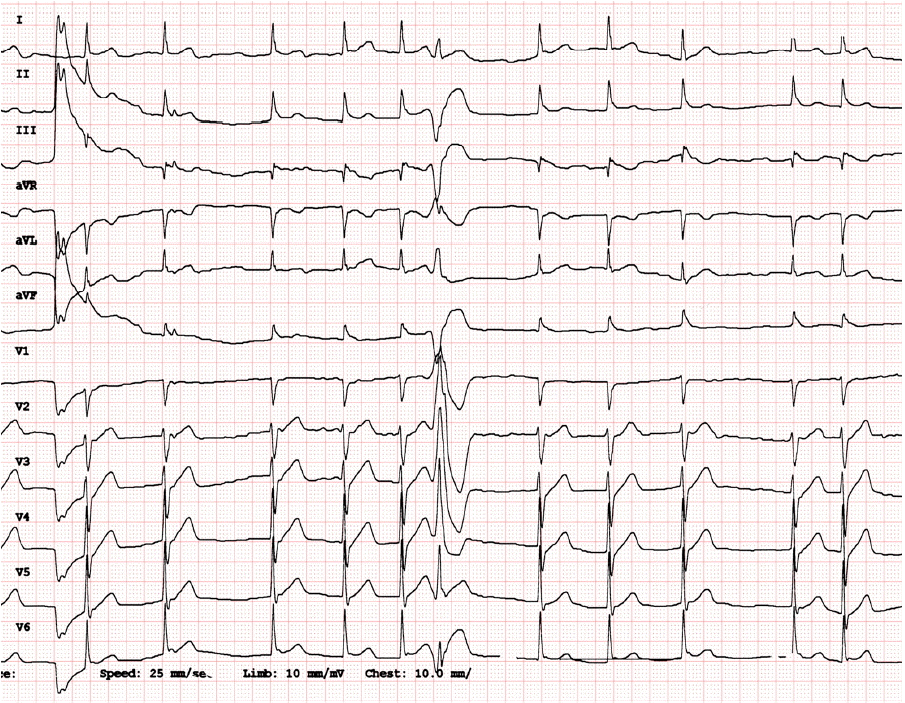
Fig. 2.
Documented episodes of VF. Continuous rhythm monitoring shows isolated monomorphic VPCs and the initiation of VF. In each episode, isolated monomorphic VPCs (arrows) with nearly identical QRS complex morphology trigger VFs. VF: ventricular fibrillation; VPCs: ventricular premature complexes.
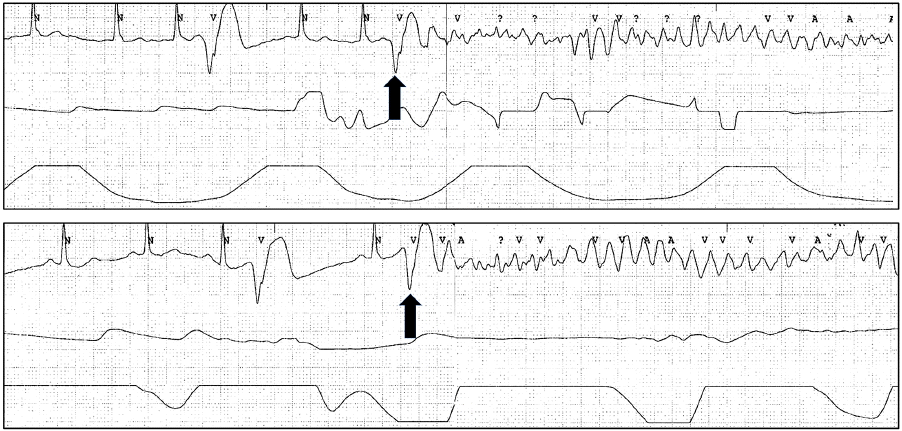
Fig. 3.
Coronary angiographic findings before and after reperfusion of infarction related artery. Right coronary angiography in the posteroanterior projection (A) before and (B) after balloon angioplasty shows a total occlusion of the small posterolateral branch, which supplies the posterior and posterolateral wall of the left ventricle (arrowheads). Right coronary angiography in the right anterior oblique 35° projection (C) before and (D) after balloon angioplasty also shows the site of acute total occlusion at the level of the left ventricular base (arrows).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download