1). Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497]. Crit Care. 2005; 9:R200–10.
2). Rozendaal FW, Spronk PE, Snellen FF, Schoen A, van Zanten AR, Foudraine NA, et al. Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: a centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med. 2009; 35:291–8.

3). Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306.

4). Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007; 106:687–95. quiz 891–2.
5). Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, et al. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesiology. 1993; 79:881–92.

6). Shapiro BA, Warren J, Egol AB, Greenbaum DM, Jacobi J, Nasraway SA, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Society of Critical Care Medicine. Crit Care Med. 1995; 23:1596–600.
7). Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008; 11(2 Suppl):S133–53.

8). Michelsen LG, Hug CC Jr. The pharmacokinetics of remifentanil. J Clin Anesth. 1996; 8:679–82.

9). Kapila A, Glass PS, Jacobs JR, Muir KT, Hermann DJ, Shiraishi M, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995; 83:968–75.

10). Pitsiu M, Wilmer A, Bodenham A, Breen D, Bach V, Bonde J, et al. Pharmacokinetics of remifentanil and its major metabolite, remifentanil acid, in ICU patients with renal impairment. Br J Anaesth. 2004; 92:493–503.
11). Steinlechner B, Dworschak M, Birkenberg B, Lang T, Schiferer A, Moritz A, et al. Low-dose remifentanil to suppress haemodynamic responses to noxious stimuli in cardiac surgery: a dose-finding study. Br J Anaesth. 2007; 98:598–603.

12). Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993; 77:1031–40.
13). Muellejans B1, López A, Cross MH, Bonome C, Morrison L, Kirkham AJ. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713]. Crit Care. 2004; 8:R1–R11.
14). Dahaba AA, Grabner T, Rehak PH, List WF, Metzler H. Remifentanil versus morphine analgesia and sedation for mechanically ventilated critically ill patients: a randomized double blind study. Anesthesiology. 2004; 101:640–6.
15). Wang JY, Winship SM, Thomas SD, Gin T, Russell GN. Induction of anaesthesia in patients with coronary artery disease: a comparison between sevoflurane-remifentanil and fentanyl-etomidate. Anaesth Intensive Care. 1999; 27:363–8.

16). Reid JE, Mirakhur RK. Bradycardia after administration of remifentanil. Br J Anaesth. 2000; 84:422–3.

17). Kurdi O, Deleuze A, Marret E, Bonnet F. Asystole during anaesthetic induction with remifentanil and sevoflurane. Br J Anaesth. 2001; 87:943.
18). Nooh N, Abdelhalim AA, Abdullah WA, Sheta SA. Effect of remifentanil on the hemodynamic responses and recovery profile of patients undergoing single jaw orthognathic surgery. Int J Oral Maxillofac Surg. 2013; 42:988–93.

19). Gesztesi Z, Mootz BL, White PF. The use of a remifentanil infusion for hemodynamic control during intracranial surgery. Anesth Analg. 1999; 89:1282–7.

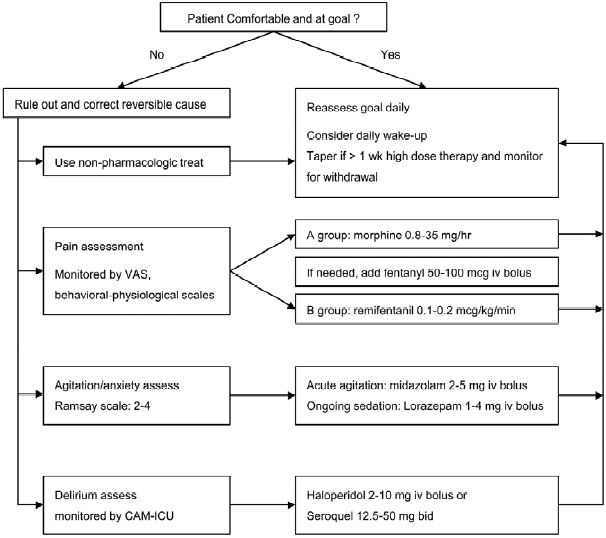
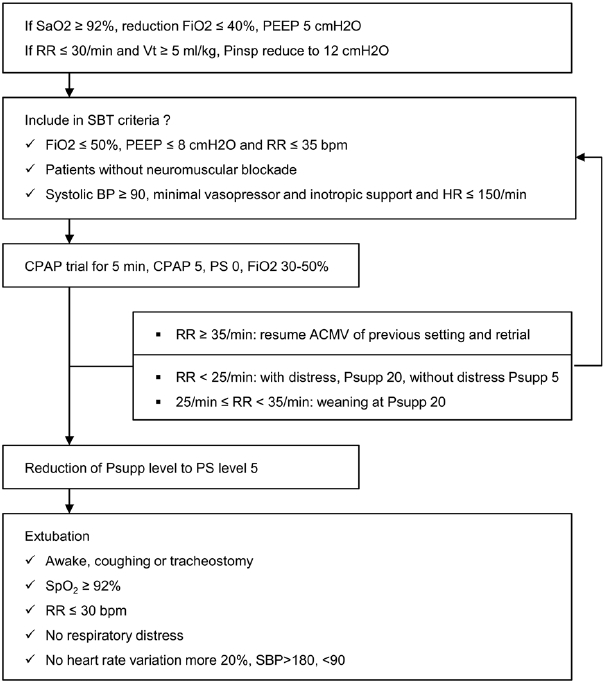
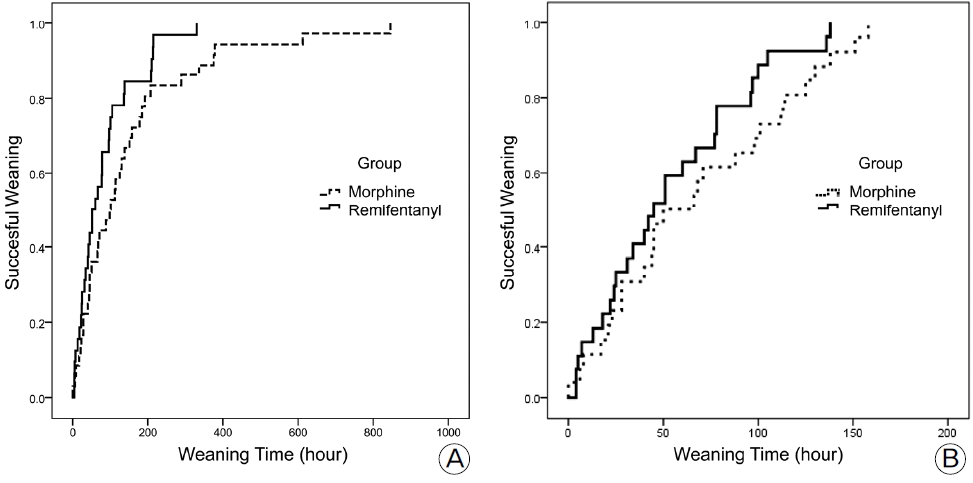
20). Carrer S1, Bocchi A, Candini M, Donegà L, Tartari S. Short term analgesia based sedation in the Intensive Care Unit: morphine vs remifentanil + morphine. Minerva Anestesiol. 2007; 73:327–32.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download