Abstract
Myocardial dysfunction can occur during severe sepsis and may accelerate in the condition of chronic decompensated heart failure. A 26-year-old female in remission from non-Hodgkin’s lymphoma presented with shock due to chronic heart failure combined with pneumonia. The patient was initially stabilized using a peripheral extracorporeal membrane oxygenator (ECMO) with antibiotics therapy, followed by left ventricular venting due to pulmonary edema that was complicated by left ventricular distension. Here, we report the successful application of ECMO to a patient with pneumonia underlying doxorubicin-induced cardiomyopathy. Although septic conditions remained unclear indication of ECMO, it might be considered a valuable therapeutic option in patients with chronic heart failure.
Extracorporeal membrane oxygenation (ECMO) is a temporary extracorporeal circulation used to support or replace cardiopulmonary function in patients with severe circulatory or respiratory failures.[1] ECMO is increasingly used for a broader range of cardiopulmonary conditions in adults, including acute respiratory distress syndrome, pulmonary embolism, acute myocardial infarction, myocarditis, peripartum cardiomyopathy accompanying cardiogenic shock and shock after heart surgery. It also serves as a bridge to transplantation of heart or lung and an important support for patients who need cardiopulmonary resuscitation.[2,3] Thus ECMO has evolved after it was originally developed for neonatal applications. According to Extracorporeal Life Support Organization’s treatment guidelines, ECMO therapy is not recommended for adult patients with severe septic shock, although it is recommended for pediatric patients with septic shock unresponsive to conventional therapy. However, effectiveness of ECMO in the management of septic shock in adult patients proved in recent studies in which ECMO was used for patients with left heart failure and septic shock.[4] In this article, we report a case of chronic heart failure accompanying pneumonia with shock that was successfully treated with ECMO.
A 26-year-old woman admitted to the intensive care unit (ICU) with shortness of breath and shock that started about six weeks ago at another hospital. The patient had a history of non-Hodgkin lymphoma two years ago and completed 8 cycles of doxorubicin-based chemotherapy (total dose of 650 mg doxorubicin). But the patient developed shortness of breath 5 months after the end of chemotherapy. The transthoracic echocardiography that was transferred from the previous hospital showed the left ventricular distension with decreased ejection fraction of 25%. Based on the history of taking doxorubicin, chronic heart failure was diagnosed at the same hospital and medication was given for that. Nonetheless, the patient developed pneumonia and subsequent shock, but the administration of both antibiotics and vasopressors did not improve her condition. That was why she was transferred to the ICU of our hospital.
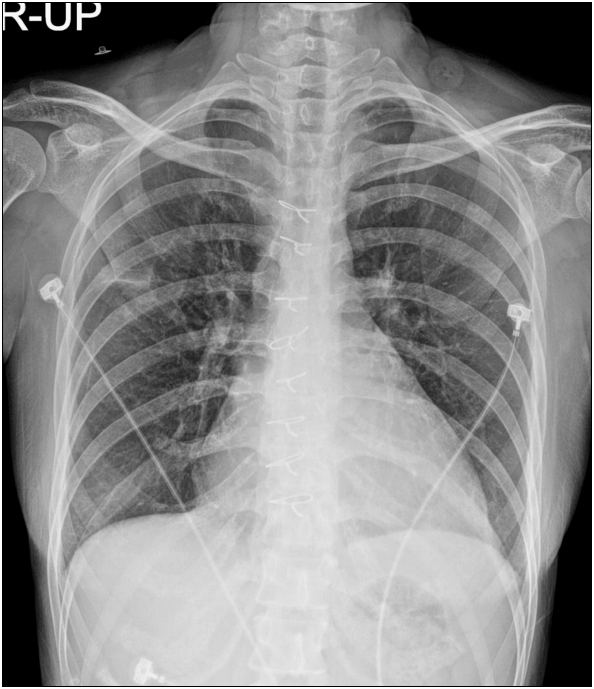
On admission, her vital signs indicated blood pressure of 79/27 mmHg, pulse rate of 133 bpm, respiratory rate of 28 bpm and temperature of 35.9°C. Auscultation revealed crackles in both lungs. When oxygen was supplied through a mask at a flow rate of 6L/min, arterial blood gas analysis showed pH 7.45, PaO2 59.0 mmHg, pCO2 25.1 mmHg and HCO3 18.6 mM/L and SpO2 91.7%. Lab findings showed a white blood cell count of 22,060/µl with 89% neutrophils, lactate of 3.75 mM/L and elevated procalcitonin level of 7.92 ng/ml. Multiple organ dysfunction, including oliguria, was also observed with elevated CK MB level of 48.31 ng/ml and troponin I level of 0.288 ng/ml. Her chest x-ray revealed cardiomegaly with pulmonary edema and consolidation of right upper lobe (Fig. 1a). An area of in-duration was observed on the upper lobe along with ground glass opacity in both lungs at chest CT scan (Fig. 1b). Based on examination results, we diagnosed cardiogenic and septic shock resulting from chronic heart failure and pneumonia and administered antibiotics and vasopressors. We also performed blood and sputum cultures to ensure associated bacterias, but no pathogenic bacteria were detected. The absence of bacteria in the blood and sputum was interpreted as effects of antibiotics that she was taking. Elevated white blood cell count, CRP and procalcitonin level however supported the clinical diagnosis of septic shock, and the administration of antibiotics and vasopressors continued. Despite the use of maximum dose of vasopressors, the patient still maintained unstable vital signs and clinical manifestations of multiorgan dysfunction syndrome with continued oliguria, blood urea nitrogen (BUN) of 22.1 mg/dl, rising creatinine level of 1.51 mg/dl, AST 191 U/L and ALT 469 U/L. Veno-arterial ECMO was therefore applied as an intervention. Her vital signs stabilized, but pulmonary edema on both sides was worsened based on chest radiography (Fig. 2a), and chest CT image still revealed left ventricular distension (Fig. 2b, Fig 2c). Transthoracic echocardiography also detected left ventricular distension and global akinesia across the left ventricular wall (Fig. 3). To prevent further worsening of pulmonary edema that might occur when the left atrial pressure rises as a result of left ventricular distension, venting operation was performed for ventricular unloading 2 days after admission. After sternotomy, a venous cannula was inserted via the right pulmonary vein and advanced via the left atrium and mitral valve into the left ventricle (Fig. 4a). The drainage cannula placed in the left ventricle was then connected with the venous cannula of ECMO to drain blood from the left ventricle (Fig. 4b).
A chest x-ray obtained 3 days after surgery demonstrated decreased pulmonary edema and smaller distension of the ventricle (Fig. 5). The patient’s vital signs became stable without the help of vasopressors. The venous cannula was removed 8 days after surgery, then the patient was put on a modified Left Ventricular Assist Device (LVAD). Transthoracic echocardiography showed the increase in left ventricular ejection fraction to 40%, and LVAD was removed next day. At 11 days after surgery, mechanical ventilation was removed. The patient was discharged in stable condition on day 28 after surgery.
ECMO is a life-saving technique in providing circulatory support for critically ill patients with cardiopulmonary failures that are unresponsive to conventional treatment.[1]
According to Extracorporeal Life Support Organization’s ECMO guidelines, contraindications to ECMO include pre-existing conditions in which anticoagulants should be avoided due to a history of hemorrhage or surgery and conditions in which cardiopulmonary failures were caused by irreversible conditions.[5] Sepsis also needs life-support intervention, but the use of ECMO in sepsis is controversial because of the hypothesis that associated bacteria of sepsis may result in cannula infection and subsequent bacteremia unresponsive to treatment.[6] In this article, ECMO was however used for the management of circulatory and respiratory failures caused by sepsis in the patient who had chronic heart failure as an irreversible condition. Veno-arterial ECMO was used to secure enough time to manage sepsis and facilitate cardiac transplantation, if necessary.
Veno-arterial ECMO is an effective circulatory support for diseases, including acute cardiogenic shock, heart failure and pulmonary embolism, but may cause pulmonary edema as a result of distension when the left ventricle lost its normal function.[7] In details, the infusion of oxygenated blood into the aorta causes an increase in afterload, which accelerates left ventricular dysfunction. Especially in cases of mitral regurgitation caused by acute decompensated heart failure, increased pressure load on the left ventricle will prompt the reflux of blood into the left atrium, forcing the left atrial pressure to rise, and pulmonary venous obstruction and pulmonary edema or hemorrhage develop subsequently. However, adequate left ventricular unloading with vent can effectively restore left ventricular function by reducing pulmonary edema and oxygen consumption.[8,9]
Based on the above mentioned guidelines, the application of veno-arterial ECMO in adults with sepsis is still subject to debate. Parkers et al.[10] stated that the majority of patients with septic shock developed myocardial failure, and septic shock was successfully treated with ECMO in patients without underlying heart disease when conventional drug therapy did not work.[11,12] As described in this case, circulatory collapse can progress quickly in patients with chronic heart failure superimposed on sepsis. In the sepsis with heart failure, the application of ECMO can be a treatment option because ECMO can allow times for infection control by preventing multiple organ injury. Also, we performed venting for left ventricular unloading along with ECMO for the patients with the failing left ventricle and achieved satisfactory outcomes. We therefore ascertain the effectiveness of ECMO as a mechanical support for ventricular function in patients with septic shock complicated by chronic heart failure and unresponsive to conventional drug therapy.
This article reports a case of pneumonia with shock in the patient who developed chronic heart failure after chemotherapy for non-Hodgkin lymphoma. The patient was successfully treated with venoarterial ECMO. We performed venting in combination with ECMO to unload the left ventricle and experienced significant improvements in left ventricular distension and pulmonary edema. After the patient’s circulatory function returned, ECMO was withdrawn. We suggest the application of ECMO as an important treatment modality for patients with chronic heart failure who developed with reversible causes such as sepsis.
REFERENCES
1). Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008; 17(Suppl 4):S41–7.

3). Almond CS, Singh TP, Gauvreau K, Piercey GE, Fynn-Thompson F, Rycus PT, et al. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the United States: analysis of data from the Organ Procurement and Transplant Network and Extracorporeal Life Support Organization Registry. Circulation. 2011; 123:2975–84.
4). Bréchot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Léger P, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013; 41:1616–26.

6). Maclaren G, Butt W. Extracorporeal membrane oxygenation and sepsis. Crit Care Resusc. 2007; 9:76–80.
7). Massetti M, Gaudino M, Crea F. How to transform peripheral extracorporeal membrane oxygenation in the simplest mid-term paracorporeal ventricular assist device. Int J Cardiol. 2013; 166:551–3.

8). Aiyagari RM, Rocchini AP, Remenapp RT, Graziano JN. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med. 2006; 34:2603–6.

9). Fumagalli R, Bombino M, Borelli M, Rossi F, Colombo V, Osculati G, et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs. 2004; 27:410–3.

10). Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984; 100:483–90.

Fig. 1.
(A) Chest radiography shows consolidation of right upper lobe. (B) Chest computed tomography scan shows consolidation of right upper lobe and ground glass opacities in both lung fields.
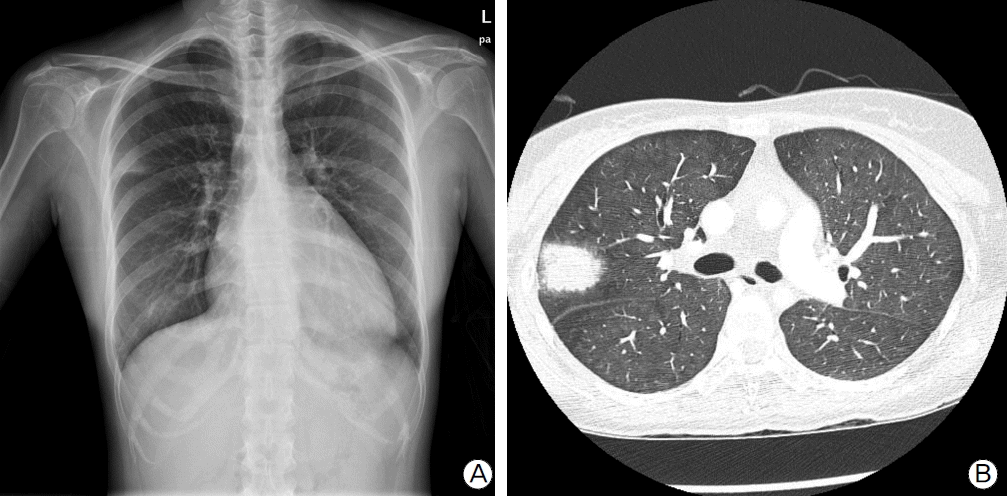
Fig. 2.
Post ECMO chest image findings. (A) Chest radiography shows extensive bilateral pulmonary infiltrations with cardiomegaly. (B) Chest computed tomography scan shows bilateral ground glass opacities with interlobular septal thickening. (C) A axial image shows a remarkable left ventricular distension.
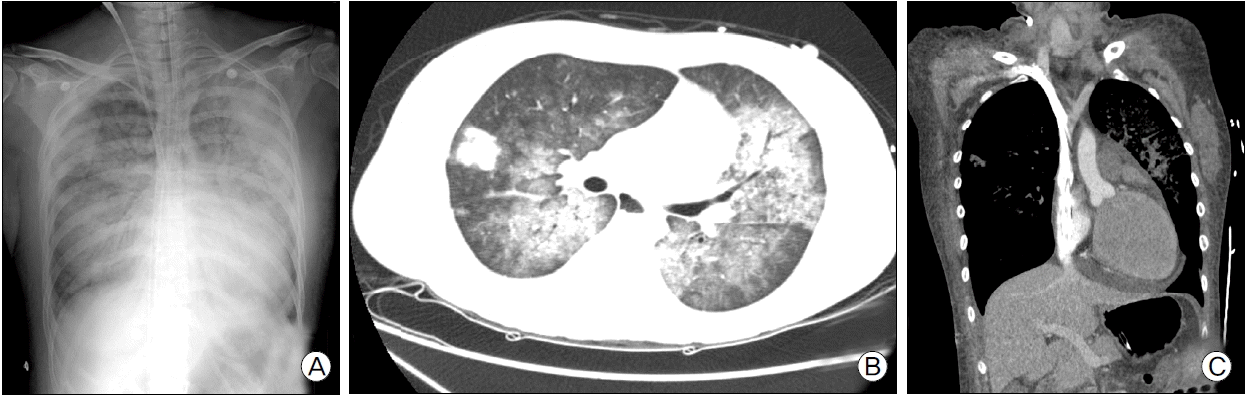
Fig. 3.
Echocardiography showed a severe LV systolic dysfunction and LV dilatation. (A) End-systolic and (B) end- diastolic period of apical four chamber view. LV: left ventricle; RV: right ventricle.
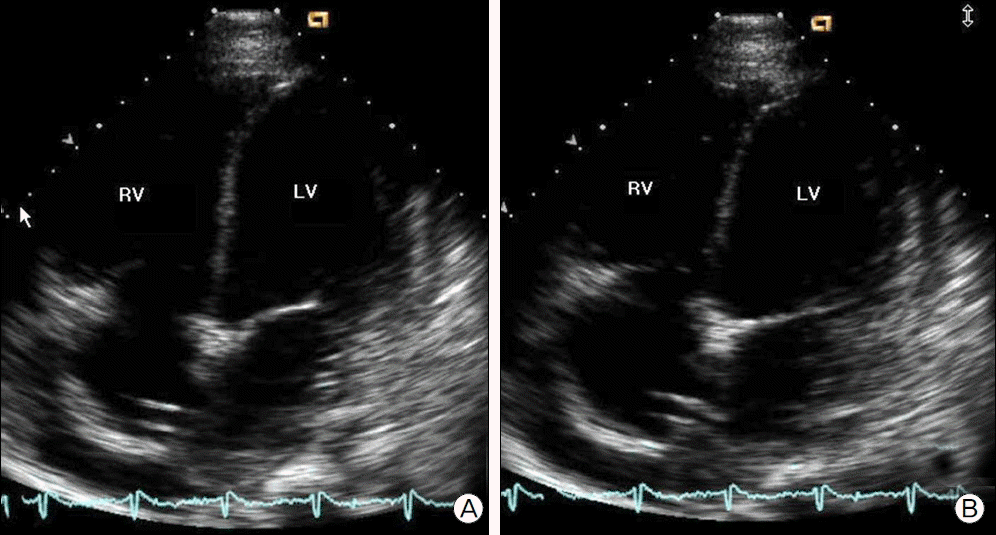
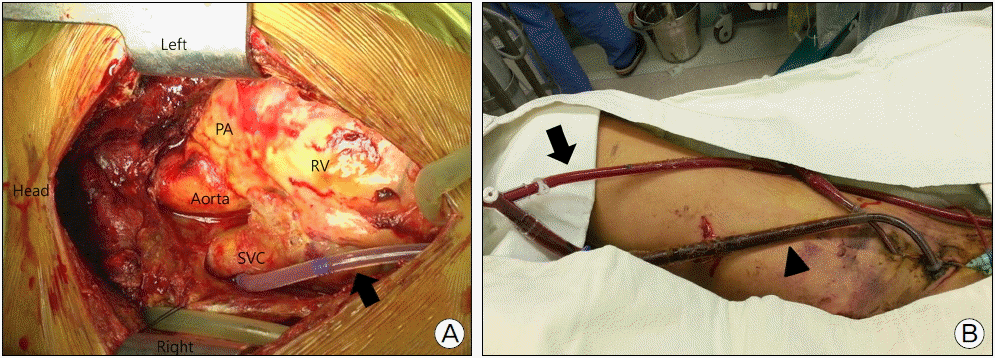
Fig. 4.
Venting operation. (A) A LV venting cannula (arrow) inserted into the right upper pulmonary vein and advanced up to the left ventricle. (B) The LV venting cannula (arrow) connected to the venous cannula (arrow head) of the ECMO circuit via a Y-shaped connector. LV: left ventricle; PA: pulmonary artery; RV: right ventricle; SVC: superior vena cava.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download