Abstract
A 25-year-old man developed tracheal stenosis due to prolonged intubation for five days. Immediately after bougienage, his left lung was not possible to ventilate and emergency tracheostomy was performed to produce ample space for airflow. Fiberoptic bronchoscopy showed that his left main bronchus was totally obstructed by sputum at the entrance of the superior and inferior lobar bronchi. Inadequate airway clearance increases the risk of infection and airway obstruction. We suggest chest physiotherapy be applied to all patients in the intensive care unit (ICU), especially patients with tracheal stenosis, due to its positive impact on pulmonary functional ability and ICU stay.
In the intensive care unit (ICU), endotracheal intubation is frequently performed to treat hypoxia due to cardiopulmonary dysfunction or to protect the airway; its complications include paratracheal trauma, bronchospasm, edema, tracheal stenosis, and tracheomalacia. Tracheal stenosis is rare; however, if it occurs, it is a difficult complication to manage. Despite the use of high volume-low pressure cuffed endotracheal tubes, the most common cause of tracheal stenosis is long-term intubation. Complications occur in 6–22% of intubated patients and among those, severe complications occur in 1–2% of patients.[1,2] Expectoration of sputum is affected by the thickness and amount of mucus and the diameter of the trachea. The smaller the airway diameter is, the thinner mucus layer required to expectorate sputum; thus, patients who have tracheal stenosis have a greater risk of infection and tracheal obstruction due to difficulties in expectorating sputum.[3]
Tracheal bougienage enlarges the diameter of the trachea in order to protect the airway and faciliates sputum expectoration. In our case, tracheal bougienage caused the accumulated sputum to move to a lower bronchus, which obstructed one lung completely, making ventilation impossible and causing hypoxia. Complete obstruction of one lung after tracheal bougienage has not been reported before. Here, the authors report their experience with emergency tracheostomy after complete obstruction of one lung due to sputum immediately after tracheal bougienage.
A 25-year-old male, height 175 cm and weight 80 kg, underwent craniotomy and epidural hematoma removal due to head trauma. He was intubated for 5 days with an intraluminal 7.5 mm cuffed endotracheal tube (Mallinckrodt Hi-Lo TM, Mallinckrodt Medical Atholen, Ireland) and admitted to the ICU for postoperative care. Chest radiography and arterial blood gas analysis (ABGA) showed no abnormal findings. Endotracheal tube cuff pressure were measured by cuff pressure manometer (MallinckrodtTM, Hennef, Germany) every 8 hours and maintained 20–22 mmHg. Endotracheal tube cuff volume was also checked daily by chest radiography. Since sedation was decreased to allow the patient to return to alertness, and no complications were identified, on postoperative day 5, we decided to extubate and transfer the patient to the general ward.
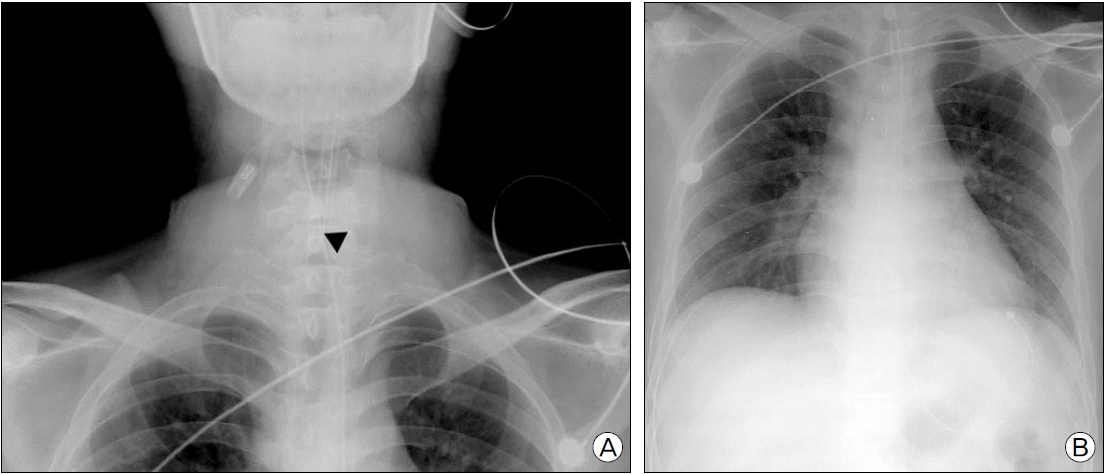
On postoperative day 25, the patient complained of dyspnea, and auscultation revealed stridor. We evaluated the patient with neck radiography (Fig. 1a), and neck computed tomography, which showed a 3 cm long area of tracheal stenosis around the thyroid gland with the narrowest transverse tracheal diameter of 5.10 mm (Fig. 1b). ABGA showed pH 7.36, PCO2 49 mmHg, PO2 65 mmHg, and BE 5.1 mM. The patient was transferred back to the ICU where he received 2 L/min of oxygen via nasal cannula; however, 5 days later the patient complained of severe dyspnea with tachypnea of 26 breath/min, cyanosis on exam, and an arterial blood gas analysis showing pH 7.33, PCO2 52 mmHg, PO2 66 mmHg, and BE 0.5 mM. Oxygen saturation was 91%, and the decision was made to attempt intubation with an intraluminal 6.0 mm size tube. Because the diameter of the main trachea was too narrow to pass the endotracheal tube (Fig. 2a), we had to fix the endotracheal tube at 18 cm at teeth and endotracheal tube cuff pressure was maintained 20 mmHg. Postintubation chest radiography showed no abnormal findings (Fig. 2b).
The patient’s symptoms of tracheal stenosis persisted, and it is known that endotracheal intubation can worsen stenosis, so tracheal bougienage was chosen to relieve the symptoms. The patient arrived to the operating room without any premedication. For total intravenous anesthesia, propofol and remifentanil were infused by a target controlled infusion pump (OrchestraTM, Fresenius Vial, France), and manual ventilation with 100% oxygen was performed for five minutes after administration of rocuronium. During surgery, the patient’s systolic and diastolic blood pressure were maintained around 130–150 mmHg and 70–90 mmHg, respectively, pulse rate was 100–110 beats/min, oxygen saturation on pulse oximetry was 99%, and bispectral index was 35–50. After extubation, tracheal bougienage via ventilating bronchoscopy was performed, and 15 mg dexamethasone was injected to prevent postoperative endotracheal edema. For reversal of neuromuscular block after surgery, 160 mg sugammadex (BridionTM, MSD, Netherlands) was used.
After removal of ventilating bronchoscopy, the patient was manually ventilated via oxygen mask. Because the patient’s spontaneous tidal volume was greater than 600 ml and the BIS was higher than 90, we confirmed the patient’s reversal from anesthesia.
At ten minutes after extubation, stridor worsened, tachypnea developed to more than 32 breaths per minute, and the patient started to use accessory respiratory muscles to breath. No breath sounds were heard in the left lung and saturation declined from 99% to 85% even with inhalation of 8 L/min of oxygen; thus, emergency tracheostomy under general anesthesia was performed to secure the airway. A 5.5 mm size tube with a cuff was used to intubate; however, the tube could not be inserted beyond 18 cm at teeth. Because we used sugammadex for reversal of neuromuscular relaxation in the previous surgery, 10 mg cisatracurium (NimbexTM, GSK, Italy), a benzylisoquinolinium non-depolarizing neuromuscular agent, was used for neuro-muscular relaxation. Afterward, we manually ventilated the patient with a respiration rate of 15 breaths per minute, at which time peak airway pressure was measured at 38 mmHg and arterial oxygen saturation on pulse oximetry increased from 85% to 95%. ABGA (FiO2 = 1.0) showed pH 7.31, PCO2 56 mmHg, PO2 99 mmHg, and BE 1.9 mM, so the respiration rate was increased to 17 breaths/min, tidal volume was increased to 700 ml, and the patient was put on mechanical ventilation. After a 7.5 mm size tracheostomy tube was inserted, the peak airway pressure was 35 mmHg, the plateau airway pressure was 29 mmHg, and no breath sounds in the left lung still were heard.
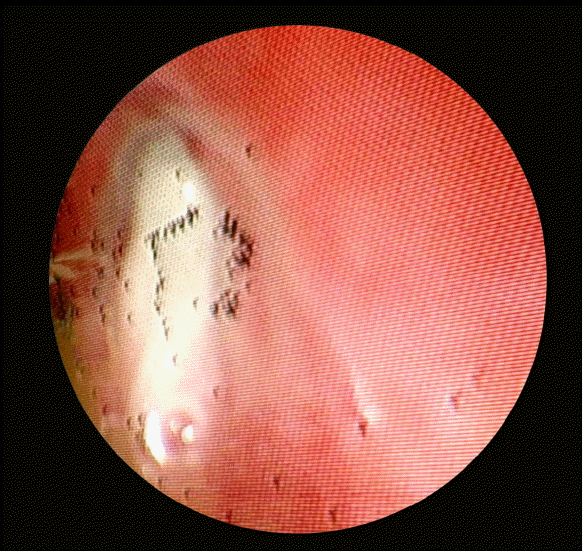
Tracheal obstruction due to edema of the left bronchus or bleeding of the tracheal tissue was suspected, so we observed the trachea via fiberoptic bronchoscopy. Even though the surgeons had suctioned massive amounts of sputum during tracheal bougienage, we discovered that the left main bronchus beyond the carina was completely obstructed by sputum, which made ventilation impossible (Fig. 3). The area from the left main bronchus to the left upper and lower lobar bronchi was completely obstructed, so for 20 minutes we suctioned out sputum as much as possible via flexible fiberoscopy. After this, breath sounds could be heard from the left lung, the peak airway pressure decreased to 22 mmHg, and the plateau airway pressure decreased to 18 mmHg. Neither dyspnea nor tachypnea were observed after recovery from anesthesia. The patient was admitted to the ICU for further close observation.
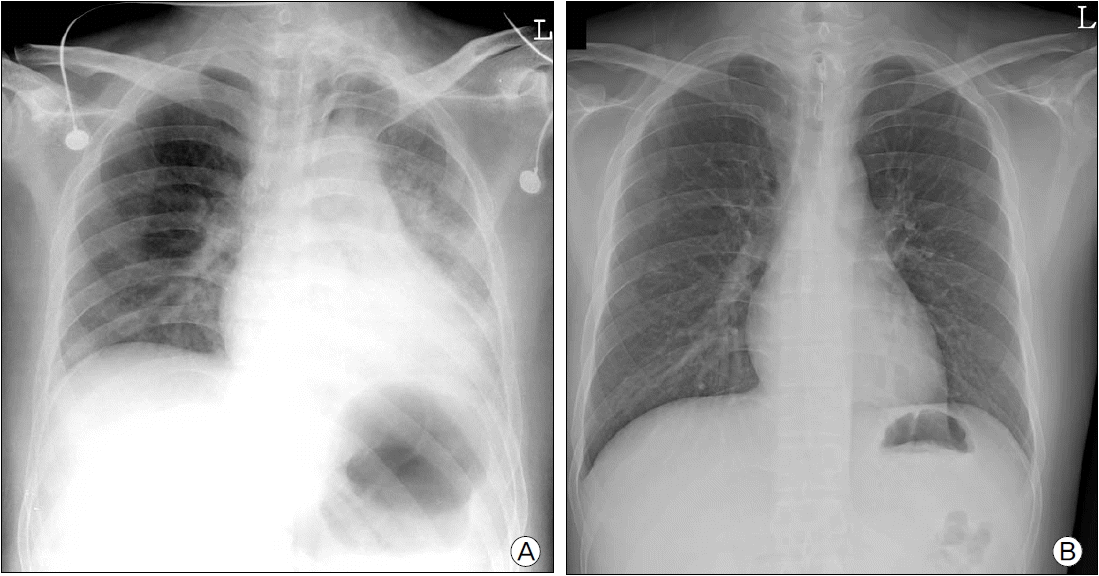
Immediate postoperative chest X-ray in the ICU showed a newly developed, ill-defined hazy infiltration in the left lung (Fig. 4a), but ABGA showed pH 7.43, PCO2 48 mmHg, PO2 119 mmHg, and BE 6.5 mM with 3 L/min oxygen supplied. On postoperative day 3, the patient’s symptoms resolved and ABGA and chest X-ray showed normal findings (Fig. 4b). The patient was transferred to the general ward and experienced no additional complications.
The most common cause of tracheal stenosis is long-term endotracheal intubation. Pressures greater than 30 mmHg applied to the tracheal mucosa lead to hypoperfusion of the capillaries and mucosal ischemia. As a result, the mucosa becomes necrotized and disappears, activating the fibrinolytic pathway and leading to commissural scarring within 3 to 6 weeks, which ultimately causes tracheal stenosis.[4] Tracheo-esophageal fistulas after endotracheal intubation caused by high endotracheal cuff pressures have been reported.[5] As a result, we decided to maintain cuff pressures under 30 mmHg and checked cuff pressures every 8 hours using a cuff pressure manometer with a goal of 20–22 mmHg. In addition, appropriate cuff volume was confirmed by chest radiography. Without risk factors for tracheal stenosis other than long-term intubation, including difficult intubation, intubation history, excessive steroid use, old age, female gender, or respiratory disease,[6] tracheal stenosis was reported in this case. As shown in this case, follow-up neck radiography until 6 weeks is necessary in long-term intubated ICU patients because tracheal fibrosis progresses until 6 weeks after extubation.
Surgical intervention such as resection and end-to-end anastomosis of the trachea is reported to be the best treatment for tracheal stenosis, but the mortality rate is up to 5% due to infection, bleeding, and restenosis. To avoid these complications, preoperative interventions such as repeated bougienage, tracheal laser cauterization, stent insertion, and mitomycin C spray are performed.[6,7] Bougienage is performed under direct view of the stenotic lesion with bronchoscopy and may result in edema due to damage from the direct contact of bronchoscopy.
Intubation lasting longer than 36 hours causes laryngoedema, which leads to tracheal obstruction in 1–17% of extubated patients in the ICU and reintubation has an associated mortality as high as 30–52.9%.[8,9] Even short-term intubation during surgery can cause laryngoedema in 2–15% of patients.[10] Based on these reports, we suspected edema after extubation to be the primary cause of respiratory failure. If edema caused by interventions for upper airway obstruction was the main reason, ventilation of both lungs should be decreased to the same degree; however, our patient had ventilation failure in only one lung, ruling out edema and bronchospasm as possible causes. Because we injected sugammadex, a novel neuromuscular block antagonist, to reverse the neuromuscular block and confirmed our patient’s consciousness, the possibility of residual neuromuscular block or residual effects of the anesthesic gas were unlikely. No active lesion was found on chest radiography done after intubation prior to surgery, so we eliminated pulmonary disease as the cause of respiratory dysfunction. Under flexible fiberoscopy, we showed that the left main bronchus was completely obstructed by sputum, which led to ventilation failure. We suspect sputum that had collected under the stenotic lesion moved to the left upper and lower lobar bronchi during tracheal bougienage, which resulted in complete obstruction.
Before surgery, the patient complained difficulty of expectoration, so we attempted to aspirate sputum via a catheter; however, the sputum beyond the stenotic lesion could not be removed. Anatomically, the suction catheter primarily enters to the right bronchus, resulting in difficult removal of sputum in the left lung and subsequent accumulation of sputum. By bougienage bronchoscopy, the accumulated mucus and sputum was accumulated in the bronchus and totally obstructed the left lung. We tried chest physiotherapy techniques such as cough, postural drainage, and chest percussion, but their ability to expectorate sputum was limited secondary to the patient’s low compliance.
Pulmonary pathology such as chronic obstructive pulmonary disease, asthma, bronchiectasis, and cystic fibrosis lead to mucus hypersecretion or dysfunction of mucus excretion, which result in bronchiolar obstruction and increased risk of infections.[11] Patients with tracheal stenosis such as in this case may have complete obstruction of one lung. Hyperinflation of the lung with pressures greater than 40 cmH2O for over 2 seconds during the inspiratory period in intubated patient promotes excretion of sputum and mucus and is reported to reduce early pulmonary complications.[12]
Coughing is the most effective treatment in chest physiotherapy. Its mechanism involves closure of the vocal cord, which causes approximately 200 cmH2O of intrathoracic pressure. As the vocal cord opens, 6–20 L/s of sudden outflow plays an important role in not only sputum excretion but also in prevention of inflammation and infection.[13] For those patients who are incapable of direct expectoration of sputum, coughing may even be effective.[14] Postural drainage induces excretion of mucus from the bronchioles to the main bronchus by gravity. According to Lanefors and Wollmer,[15] excretion is induced not only by gravity, but also by increased ventilation in dependent regions of the lung, which allows higher airflow. Because postural drainage is necessary to maintain the same position from at least 3 minutes to more than 15 minutes, it has its limitations in positions such as Trendelenburg reduce tidal volume and functional residual volume, which increases the work of breathing. But if postural drainage is used with other chest physiotherapy techniques, it could be applied to patients effectively.[16] Standardizing protocols of chest percussion to speeds of 3–6 Hz for 10–20 minutes causes air turbulence and compression so as to not only mobilize airway secretions but also to normalize the perfusion to ventilation ratio and functional residual capacity.[14,17] Other methods, including high frequency chest wall compression, positive expiratory pressure therapy, and mucolytic therapy such as n-acetyl cysteine, are used to decrease the amount of mucus. Short-term research confirms that chest physiotherapy helps mobilize airway secretions,[18] and has a positive impact on pulmonary functional ability, shortening ICU stays.[19,20]
Intubated patients in the ICU should always be monitored for the development of tracheal stenosis, as the accumulated sputum from tracheal stenosis can decrease ventilation by obstructing the trachea. We experienced a case of acute tracheal obstruction due to accumulated sputum that moved to the lower trachea after tracheal bougienage, which was undertaken in an effort to widen the tracheal diameter. We suggest chest physiotherapy be applied widely and aggressively to prevent mucus accumulation and tracheal obstruction for patients with respiratory depression in the ICU.
REFERENCES
1). Sarper A, Ayten A, Eser I, Ozbudak O, Demircan A. Tracheal stenosis after tracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J. 2005; 32:154–8.
2). Rahman NA, Fruchter O, Shitrit D, Fox BD, Kramer MR. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg. 2010; 5:2.

3). Ntoumenopoulos G, Shannon H, Main E. Do commonly used ventilator settings for mechanically ventilated adults have the potential to embed secretions or promote clearance? Respir Care. 2011; 56:1887–92.

4). Puchalski J, Musani AI. Tracheobronchial stenosis: causes and advances in management. Clin Chest Med. 2013; 34:557–67.
5). Kim MS, Koh EJ, Choi HY. Occurrence of acquired tracheoesophageal fistula due to excess endotracheal tube cuff volumes - A Case Report-. Korean J Crit Care Med. 2013; 28:146–51.
6). Zias N, Chroneou A, Tabba MK, Gonzalez AV, Gray AW, Lamb CR, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med. 2008; 8:18.

7). Bagheri R, Majidi M, Khadivi E, attar AS, Tabari A. Outcome of surgical treatment for proximal long segment post intubation tracheal stenosis. J Cardiothorac Surg. 2013; 8:35.

8). Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent post-extubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care. 2007; 11:R72.

9). Cavallone LF, Vannucci A. Extubation of the difficult airway and extubation failure. Anesth Analg. 2013; 116:368–83.

10). Chaudhary K, Anand R, Girdhar KK, Bhalotra A, Manchanda G. Airway obstruction following intubation using a bonfils rigid intubating fiberscope and polyvinylchloride tracheal tube. J Anaesthesiol Clin Pharmacol. 2013; 29:287–9.

11). Nadel JA. Mucous hypersecretion and relationship to cough. Pulm Pharmacol Ther. 2013; 26:510–3.

12). Hodgson C, Denehy L, Ntoumenopoulos G, Santamaria J, Carroll S. An investigation of the early effects of manual lung hyperinflation in critically ill patients. Anaesth Intensive Care. 2000; 28:255–61.

13). Mauroy B, Fausser C, Pelca D, Merckx J, Flaud P. Toward the modeling of mucus draining from the human lung: role of the geometry of the airway tree. Phys Biol. 2011; 8:056006.

14). van der Schans CP. Conventional chest physical therapy for obstructive lung disease. Respir Care. 2007; 52:1198–206. discussion 1206–9.
15). Lannefors L, Wollmer P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: a comparison between postural drainage, PEP and physical exercise. Eur Respir J. 1992; 5:748–53.
16). Fink JB. Positioning versus postural drainage. Respir Care. 2002; 47:769–77.
17). Cross J, Elender F. Findings from the MATREX study: a treatment protocol for the delivery of manual chest therapy in respiratory care. Respir Care. 2012; 57:1263–6.

18). McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129(1 Suppl):250S–9S.
19). Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013; 144:825–47.
Fig. 1.
(A) Neck X-ray 25 days after extubation shows segmental narrowing of the upper trachea. (B) Neck CT shows focal tracheal narrowing with wall thickening around the lower thyroid pole (black arrow head). The narrowest transverse diameter of the trachea is 5.10 mm.
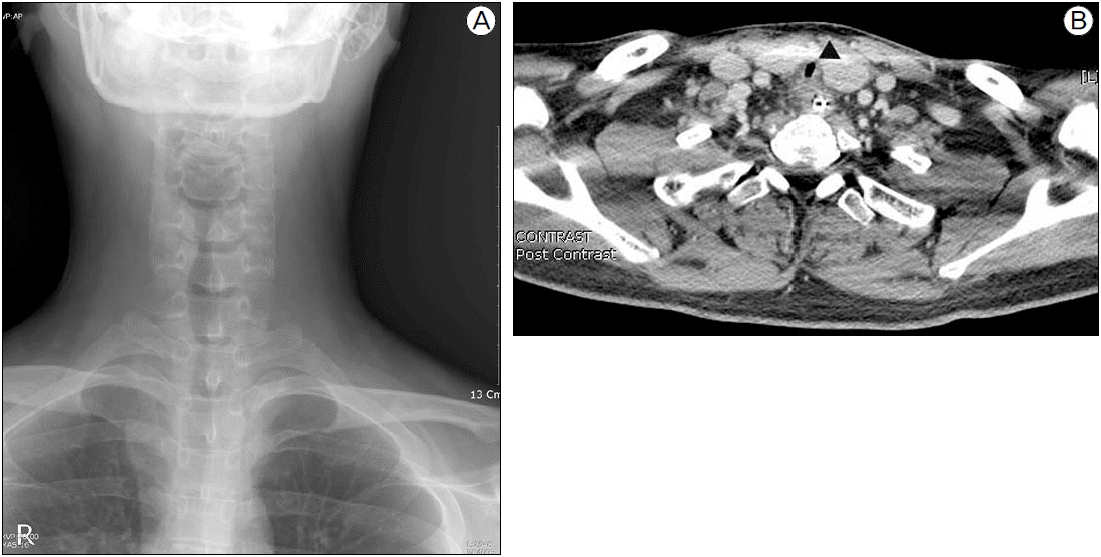
Fig. 2.
(A) Preoperative neck X-ray in the intensive care unit shows endotracheal tube is not advanced due to the tracheal stenosis (black arrow head). (B) Preoperative chest X-ray in the intensive care unit shows the endotracheal tube is inserted, and there are no active lesions in the lung.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download