Abstract
Objectives
We propose an automatic breast mass detection algorithm in three-dimensional (3D) ultrasound (US) images using the Hough transform technique.
Methods
One hundred twenty-five cropped images containing 68 benign and 60 malignant masses are acquired with clinical diagnosis by an experienced radiologist. The 3D US images are masked, subsampled, contrast-adjusted, and median-filtered as preprocessing steps before the Hough transform is used. Thereafter, we perform 3D Hough transform to detect spherical hyperplanes in 3D US breast image volumes, generate Hough spheres, and sort them in the order of votes. In order to reduce the number of the false positives in the breast mass detection algorithm, the Hough sphere with a mean or grey level value of the centroid higher than the mean of the 3D US image is excluded, and the remaining Hough sphere is converted into a circumscribing parallelepiped cube as breast mass lesion candidates. Finally, we examine whether or not the generated Hough cubes were overlapping each other geometrically, and the resulting Hough cubes are suggested as detected breast mass candidates.
Results
An automatic breast mass detection algorithm is applied with mass detection sensitivity of 96.1% at 0.84 false positives per case, quite comparable to the results in previous research, and we note that in the case of malignant breast mass detection, every malignant mass is detected with false positives per case at a rate of 0.62.
It is widely accepted that digital mammograms or tomosynthesis mammograms [123] are associated with quite limited use in cases of small breast masses within dense breast tissues owing to the obscure visualization of small masses when using this method, whereas ultrasonography (US) [456] has been clinically proven to be capable of detecting masses even in dense breasts and is widely used in Asia for the detection as well as the classification of breast masses. Typically, handheld probes for US scanners have been used for breast cancer screening with conventional US imaging devices having disadvantages such as operator dependence and poor image quality levels. It should also be noted that it usually takes a long time to perform conventional breast ultrasonography for breast cancer screening with some breast regions still left unscanned; thus, recently various studies have been reported regarding the development of the three-dimensional (3D) breast scanning devices to overcome these shortcomings [78].
With 3D US scanners, the entire breast image volume can be acquired easily and repeatedly in a relatively short time practically without non-scanning breast regions [891011]. However, it should be noted that a considerable amount of US image data upon each screening can exhaust radiologists when faced with the diagnosis of even one case. To reduce the required diagnostic effort using 3D US scanning devices, a computer-aided detection (CAD) system can suggest mass candidates to enhance radiologists’ screening speed and accuracy as a preliminary step in the diagnosis process [12131415161718].
Recently, various studies have been reported regarding computer-assisted detection and classification using various methods. Ikedo et al. [4] reported a fully automatic lesion detection technique using the Canny edge detector [19]. Kim et al. [14] reported a computer-aided detection algorithm using the adjusted Otsu threshold and a support vector machine classifier. Yap et al. [20] reported a lesion detection algorithm which uses hybrid filtering, multifractal processing, and a thresholding segmentation method. Moon and his colleagues reported many successful works using textural, speckle and morphological features with ultrasound elasticity images [18] and automated 3D breast ultrasound (ABUS) images [1517].
In this paper, we propose an automatic mass detection algorithm using the Hough transform technique.
The 3D US volumetric images were acquired using an ultrasound scanner (Voluson 530D; Madison, Seoul, Korea) by Seoul National University Hospital, Seoul, Korea, and were diagnosed by an experienced radiologist. Images were collected for various patient ages, types of mass lesions, shapes, and positions, and converted into a standardized 3D multiplanar format that simultaneously displays coronal, sagittal, and axial views of the breast masses. The volume of interest (VOI) regions around pixel dimensions of 125 × 125 × 125 including the specified breast masses were cropped for analytical use. Finally, 68 benign and 60 malignant masses from 125 patients were confirmed with histopathological examinations.
The detection algorithms presented in this paper were written with Visual C++ using the Visualization Toolkit (VTK) library for the visualization of a US image volume and the National Library of Medicine Insight Segmentation and Registration Toolkit (ITK) library for general image segmentation. ITK is an open-source library consisting of implementations for a variety of segmentation and registration algorithms. We used the ITK and VTK open-source software toolkits as a basis for the implementation of the mass lesion detection processes. We developed automatic mass detection algorithms for 3D image data.
It is well-known that most breast masses are observed to circular on the 2D US plane, with the inside darker than the surrounding regions. To extract the spherical feature from the 3D US image volume easily, as preprocessing steps before the Hough transform, 3D US images were masked, subsampled, contrast-adjusted, and median-filtered [4]. The overall pre-processing method applied to each 3D US image outlined in Figure 1 and is briefly described below.
First, voxels not belonging to the scanned image volume or showing grey level values outside the dynamic range of actual 3D US data are masked out in order to reduce the computing overhead and avoid undesirable results from the masked voxels.
It is often advantageous to apply a subsampling process to remove the sharp edges from speckle noise in order to enhance the accuracy of edge detection using the Canny edge detection algorithm [419]. In this paper, we subsampled each 3D US image by 1/2.
To perform the Hough transform independent of the individual scanning condition, we normalize the dynamic range of the grey-level distributions of the subsampled 3D US images to ensure that the mean and standard deviation values of each US image were 128 and 55, respectively.
A median filter with a window of 5 × 5 × 5 pixels is again applied to reduce the remnant speckle noise from the image while preserving the edge patterns.
Thereafter, the 3D Hough transform is utilized to find spherical shape, and finally the mass position is located. The Hough transform is widely used for the detection of various types of figures, such as curves, by exploiting the duality between points on a curve and its parameters. Duda and Hart [21] proposed this concept in 1972 to detect lines and curves in pictures, and Ballard [22] generalized the concept to detect an arbitrary shape.
In this paper, the 3D Hough transform is adopted to detect spherical figures in breast image volumes. Hough spheres are generated and sorted in the order of votes. In our study, 16 Hough spheres after the Hough transform were selected and converted into circumscribing parallelepiped cubes for each 3D US image volume as breast mass lesion candidates. In order to reduce the false positive rate of the breast mass detection algorithm, Hough spheres with means or grey level values of their centroids higher than the mean of VOI were excluded.
The Hough cubes were then compared with each other if two of them were geometrically overlapped. If this was not the case, we compute their weight functions {Ωi} with the formula below.
Here, mi denotes the mean grey level value of voxels within the i-th Hough cube, cmij is the mean grey level value of voxels on line (lij) between the center points of the i-th and j-th Hough cubes, Lij is the length of lij described above, and Δcσi is the standard deviation of the grey level values of voxels on lij. Subsequently, we select and combine the two lowest weight values of the Hough cubes. The resulting Hough cubes are suggested for the detected breast mass candidates [20].
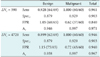
The detection results using the Hough transform technique are shown in Table 1. The proposed detection scheme for breast masses using 3D US images was evaluated using our database. In this paper, to estimate the sensitivity (Sens) of the proposed algorithm, the detected region is considered to be a true positive if the overlapped voxel volume between the detected mass candidate and the clinical gold standard exceeds ΔVc. If we consider the automated breast mass detection results as a second observer, it is often reasonable that the suggestion of some overlapped mass candidates to a clinician is sufficient to help with the early screening of breast cancer by focusing his attention on a region in close proximity to the actual breast mass.
Among 69 benign and 60 malignant breast masses, 64 benign and 60 malignant masses were successfully detected using the criterion of ΔVc = 590, and the detection sensitivity and specificity were found to be 96.1% and 90.3% at a false positive rate of 0.84, comparable to the results in other studies [41420].
In this paper, we suggest a parallelepiped-type cube as a candidate for breast mass lesions and obtain a FP candidate of a single large cube instead of many FP candidates at the limit of specificity approaches zero. Therefore, the conventional definition of specificity is not appropriate for our mass detection algorithm. To estimate the specificity and detection efficiency of the proposed algorithm using an ROC analysis, we newly define the volumetric specificity (SpecV) as
where vcandidate-gold_standard is defined as the sum of the mass candidate’s voxels not belonging to the gold standard and vwhole-gold_standard is the sum of the voxels of the entire volume not belonging to the gold standard. Using the alternative definition of specificity, we obtain the entire voxel set filled with TP/FP voxels without any TN/FN voxels in the limited case of infinitely many candidates, implying that we identify all of the gold standards of the breast masses. However, it is important to note breast masses clearly regardless of the possibility of the absence of a breast mass, giving the asymptotic results of SpecV = 0.
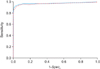
By increasing the number of Hough cubes as mass candidates, we obtain the ROC curve for the proposed mass detection algorithm using the 3D Hough transform. As shown in Figure 2, the value of the area (Az) under the ROC curve is 0.971 with ΔVc = 590, quite comparable to the results in previous studies [4] and indicating that the proposed algorithm is promising for rapid and efficient breast mass screening. In order to examine the ΔVc dependence of the ROC curve analysis, we increased ΔVc to 4720, finding that Az is not greatly reduced, still showing sensitivity of 100% with regard to malignant breast mass detection.
It is especially notable that the sensitivity of malignant breast mass detection is fairly well enhanced to 100% at 0.62 false positives per case [1420]. This may indicate that an ultrasound volume scanner such as an automated breast ultrasound system using the proposed detection algorithm can provide sufficiently accurate and economically efficient annual breast cancer screening with enhanced detection sensitivity for malignant breast masses.
It is well-known that the US images are associated with subjective interpretations even when experienced radiologists are involved, as well as inconsistent image quality levels and user-dependent reproducibility. An automatic breast mass detection algorithm can help radiologists to enhance their diagnostic accuracy for breast cancer screening. It should be noted that further research regarding the validation with a larger data set is necessary. This will be discussed later in a future study.
In conclusion, an automatic breast mass detection algorithm using the Hough transform technique is proposed for 3D US images and its mass detection efficiency is investigated by conducting a ROC analysis. The algorithm includes masking, subsampling, contrast-adjustment, and median-filter steps as preprocessing steps in order to detect spherical edges using the 3D Hough transform effectively. The 3D Hough transform is very simple, fast, and quite accurate when used to detect well-defined geometric shapes from images compared to the other complicated detection algorithms used elsewhere. The proposed algorithm provides mass detection sensitivity of 96.1% at a rate of 0.84 false positives per case, quite comparable to the outcomes in previous studies, with malignant masses detected with a false positive rate of 0.62.
Figures and Tables
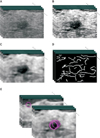
Figure 1
Illustration of pre-processing steps on 3D US images. (A) The original image. (B) RDCA is applied on (A). (C) Then, median filter is applied on (B). (D) Then, Canny edge is detected. (E) Finally, 3D Hough transform is performed on (D).
Acknowledgments
The first author wishes to acknowledge the co-authors for their co-operative support, patience, and fruitful discussions. We would like to acknowledge the financial support from the Converging Research Center Program (Grant 2013K000436) of the MSIP and the R&D Convergence Program (Grant CAP-13-3-KERI) of NST (National Research Council of Science & Technology) of Republic of Korea.
References
1. Chan HP, Wei J, Sahiner B, Rafferty EA, Wu T, Roubidoux MA, et al. Computer-aided detection system for breast masses on digital tomosynthesis mammograms: preliminary experience. Radiology. 2005; 237(3):1075–1080.

2. Singh S, Tourassi GD, Baker JA, Samei E, Lo JY. Automated breast mass detection in 3D reconstructed tomosynthesis volumes: a featureless approach. Med Phys. 2008; 35(8):3626–3636.

3. Oh WV, Kim K, Kim YJ, Kang H, Ro J, Moon W. Detection of microcalcifications in digital mammograms using foveal method. J Korean Soc Med Inform. 2009; 15(1):165–172.

4. Ikedo Y, Fukuoka D, Hara T, Fujita H, Takada E, Endo T, et al. Development of a fully automatic scheme for detection of masses in whole breast ultrasound images. Med Phys. 2007; 34(11):4378–4388.

5. Ikedo Y, Fukuoka D, Hara T, Fujita H, Takada E, Endo T, et al. Computerized mass detection in whole breast ultrasound images: reduction of false positives using bilateral subtraction technique. Proc SPIE Int Soc Opt Eng. 2007; 6514:65141.

6. Yu D, Lee S, Lee JW, Kim S. Automatic lesion detection and segmentation algorithm on 2D breast ultrasound images. Proc SPIE Int Soc Opt Eng. 2011; 7963:79631.

7. Son SH, Simonov N, Kim HJ, Lee JM, Jeon SI. Preclinical prototype development of a microwave tomography system for breast cancer detection. ETRI J. 2010; 32(6):901–910.

8. Chang JM, Moon WK, Cho N, Park JS, Kim SJ. Radiologists' performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur J Radiol. 2011; 78(1):99–103.

9. Chou YH, Tiu CM, Chen J, Chang RF. Automated full-field breast ultrasonography: the past and the present. J Med Ultrasound. 2007; 15(1):31–44.

10. Chang JM, Moon WK, Cho N, Park JS, Kim SJ. Breast cancers initially detected by hand-held ultrasound: detection performance of radiologists using automated breast ultrasound data. Acta Radiol. 2011; 52(1):8–14.

11. Clauser P, Londero V, Como G, Girometti R, Bazzocchi M, Zuiani C. Comparison between different imaging techniques in the evaluation of malignant breast lesions: can 3D ultrasound be useful? Radiol Med. 2014; 119(4):240–248.

12. Horsch K, Giger ML, Vyborny CJ, Lan L, Mendelson EB, Hendrick RE. Classification of breast lesions with multimodality computer-aided diagnosis: observer study results on an independent clinical data set. Radiology. 2006; 240(2):357–368.

13. Sahiner B, Chan HP, Roubidoux MA, Hadjiiski LM, Helvie MA, Paramagul C, et al. Malignant and benign breast masses on 3D US volumetric images: effect of computer-aided diagnosis on radiologist accuracy. Radiology. 2007; 242(3):716–724.

14. Kim JH, Cha JH, Kim N, Chang Y, Ko MS, Choi YW, et al. Computer-aided detection system for masses in automated whole breast ultrasonography: development and evaluation of the effectiveness. Ultrasonography. 2014; 33(2):105–115.

15. Moon WK, Lo CM, Chang JM, Huang CS, Chen JH, Chang RF. Computer-aided classification of breast masses using speckle features of automated breast ultrasound images. Med Phys. 2012; 39(10):6465–6473.

16. Jalalian A, Mashohor SB, Mahmud HR, Saripan MI, Ramli AR, Karasfi B. Computer-aided detection/diagnosis of breast cancer in mammography and ultrasound: a review. Clin Imaging. 2013; 37(3):420–426.

17. Moon WK, Shen YW, Huang CS, Chiang LR, Chang RF. Computer-aided diagnosis for the classification of breast masses in automated whole breast ultrasound images. Ultrasound Med Biol. 2011; 37(4):539–548.

18. Moon WK, Choi JW, Cho N, Park SH, Chang JM, Jang M, et al. Computer-aided analysis of ultrasound elasticity images for classification of benign and malignant breast masses. AJR Am J Roentgenol. 2010; 195(6):1460–1465.

19. Canny J. A computational approach to edge detection. IEEE Trans Pattern Anal Mach Intell. 1986; 8(6):679–698.

20. Yap MH, Edirisinghe EA, Bez HE. A novel algorithm for initial lesion detection in ultrasound breast images. J Appl Clin Med Phys. 2008; 9(4):2741.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download