Abstract
Tricuspid regurgitation (TR) has long been neglected based on the false belief that it is substantially rare in prevalence and is not so important in determining prognosis. Recent consecutive publications refuted this concept surrounding TR, and now we are contemplating this entity from different point of view. In this review, we mainly focus on isolated form of severe TR. In our daily clinical practice, however, patients with problems in more than one valve are more frequently encountered. Hence, we briefly touch on the results of severe TR surgery with or without left side valve operations here and there, as well.
Tricuspid regurgitation (TR) has long been ignored by many cardiologists in comparison with the interest expressed to mitral regurgitation. A PubMed database search from January 1900 to September 2012 using the term "mitral regurgitation" unearthed 24013 papers, whereas "tricuspid regurgitation" generated only 4294. This imbalance probably stems from the false belief that TR is not clinically significant and that significant TR, usually defined as TR of more than mild degree, is not a frequent finding. Furthermore, since TR is frequently detected in association with left side valve disease, its clinical manifestations tend to be predominated by underlying left side valve disease. In this respect, TR has been regarded as an "orphan disease" or as a "forgotten disease". The following provides a representative example of "ignored" TR. A 50-year-old man diagnosed with infective endocarditis of the tricuspid valve was reported in 2001.1) He had been a registered intravenous drug abuser for a considerable time. Due to concerns about the potential of repeat infective endocarditis caused by illegal drug injection, total tricuspid valvectomy was conducted without insertion of a bioprosthetic or prosthetic tricuspid valve. Somewhat surprisingly, the authors reported that he survived more than 10 years after tricuspid velvectomy.1) However, recent studies have consistently shown that significant TR that is not artificially created is not a rare disease, and that its prevalence is progressively growing in specific settings, for example, in patients who have previously undergone left side valve surgery with or without combined left side valve dysfunction.2-6) If untreated, TR patients progressively deteriorate and develop refractory heart failure symptoms related to right ventricular (RV) dysfunction, eventually resulting in death,3)7-9) irrespective of the presence of left ventricular dysfunction or pulmonary hypertension.9) Thanks to recent growing interest in TR, we can now understand that TR should not be viewed as a benign disease and that it can exert a significant influence on outcome. Thus, we believe that TR undoubtedly deserves to be treated seriously and should not be neglected. Today, diagnostic techniques like echocardiography and cardiac magnetic resonance imaging have been well developed, and allow for the early detection of disease and prompt establishment of appropriate treatment. In this era of increasing awareness of the importance of TR, the authors considered that an overview of currently available evidence on TR management and prognosis might be of value.
This review mainly focuses on the isolated form of TR, which is defined as TR in the setting of no concomitant left side valve lesions, because it was believed that such a homogenous TR patient population would enable a better understanding of the therapeutic and prognostic implications of TR. However, in daily clinical practice, patients with problems in more than one valve are more frequently encountered. Hence, this review also briefly touches on the study results dealing with patients with multi-valve diseases.
TR is not an uncommon finding in patients undergoing mitral or combined mitral/aortic valve surgery.10)11) In the past, it was generally believed that TR can regress after successful mitral or combined mitral/aortic surgery by reducing RV volume and/or pressure overload.12) However, this is not always true and sometimes, not infrequently, TR progresses after index surgery without any evidence of left side valve dysfunction.13)14) TR can appear de novo during postoperative follow-up, as well. Incidences of late significant TR development after left side valve surgery vary from 16% to 67%, depending on study population, the definition of significant TR used, and most importantly on follow-up duration.3)15)16) Matsuyama et al.16) reported TR incidence of 16% after mitral valve surgery, but the study duration was only intermediate (mean 8.2 years). On the other hand, Porter et al.14) reported an even higher incidence of TR (67%) after left side valve surgery for a longer follow-up (mean 11.6 years). However, the number of patients enrolled in their study was only 65. We previously reported in 335 patients without significant TR immediately after corrective surgery for left side valve disease that over a mean follow-up duration of 11.6 years, significant TR developed in 90 patients (26.9%).3) Interestingly, we also found that preoperative atrial fibrillation was the only risk factor of the late development of significant TR.3) Similar results have been reported by other groups and ours thereafter.2)14)16)17) Given the clinical implications of atrial fibrillation on the late development of significant TR, concomitant maze operation was highlighted as an effective preventive strategy. The first study on this topic was published in Circulation in 2005 by our group, where the maze operation could reduce the risk of late significant TR by as much as 79%.2) Of note, the objective maintenance of atrial activity, as demonstrated by Doppler echocardiography, was found to independently benefit the maze operation beyond the simple elimination of atrial fibrillation.2) A few years later, the favorable effect of maze operation on the prevention of late TR development was again shown by Je et al.15) and Stulak et al.18) In particular, Je et al.15) eloquently showed that the maze operation was superior to tricuspid valve annuloplasty in the prevention of late TR development.
It is well accepted that significant TR development with or without left side valve disease badly influences long-term morbidity and mortality.9) Even when we excluded patients with prior left side valve surgery, late TR development was again found to be closely associated with poor event-free survival.3) Apart from survival, quality of life is also severely impaired in patients with significant TR after left side valve surgery. Groves et al.8) showed that patients with isolated severe TR after left side valve surgery exhibit a significant reduction in exercise duration, maximal oxygen consumption, and anaerobic threshold as compared with those without severe TR, although all patients had good left ventricular systolic performance and normal prosthetic valve function.
We believe that more than 10 years of follow-up is required for the early identification of significant TR occurrence, because event-free survival curves according to the presence or absence of significant TR start to diverge from at least 10 years after index left side valve surgery, as shown in Fig. 1. Therefore, it is highly recommended that patients who undergo left side valve surgery should be closely monitored for more than 10 years after index surgery, preferably with periodic echocardiographic examinations.
The pathogenesis of TR in patients with concomitant mitral valve disease is complex and multifactorial. Detailed description of this entity is beyond the scope of this review. In short, TR in patients with concomitant mitral valve disease is mostly "functional" in origin, and is considered to be caused by passive downstream elevation in left heart pressures, generating eventually tricuspid annular dilation and the tethering of tricuspid leaflets induced by RV enlargement.8)19-21) This may also be true in patients with late TR after left side valve surgery.3)9)19-21) Based on this concept, tricuspid annuloplasty with or without an annular ring has been widely conducted in the hope of reducing tricuspid annular diameter and ultimately of eliminating significant TR. However, these two mechanisms cannot always account for TR development and, in fact, earlier studies found that tricuspid annuloplasty per se is not always effective at reducing or taking away TR.2)22)23)
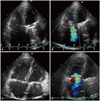
Taking the case of the 57-year-old female patient presented in Fig. 2, who presented at the outpatient clinic with exertional dyspnea [New York Heart Association (NYHA) functional class II]. She had undergone mitral valve replacement with a mechanical valve 14 years previously. Preoperative echocardiography revealed severe TR, and thus, corrective surgery was performed to eliminate the TR. On postoperative follow-up echocardiography, tricuspid annuloplasty with an annular ring was clearly shown (Fig. 2C), however severe TR was still there on Doppler echocardiography. This case strongly suggests that a factor other than tricuspid annular dilation is implicated in TR development. Fukuda et al.22) demonstrated that tricuspid valve tethering predicts residual TR after tricuspid annuloplasty, and in another study, the same group showed that right and left ventricular functions superimposed on tricuspid valve tethering are also important determinants for residual TR after tricuspid annuloplasty.23) RV remodeling pattern is another important, 'unrealized' mechanistic factor underlying the development of TR.24) Recently, three-dimensional echocardiography was introduced to provide more comprehensive, detailed information on geometrical changes in the tricuspid valve complex before and after tricuspid annuloplasty, and may have therapeutic implications for TR surgery.25)
In isolated TR, the RV progresses to a volume-overloaded state, and concurrently, both right atrial dilation and right atrial pressure elevation take place. From an anatomical viewpoint, right atrium is a common room, through which systemic venous returns from the inferior vena cava and superior vena cava move into the pulmonary circulation. Since right atrial pressure goes up over time in the setting of severe TR, the superior vena cava becomes dilated, and leads to jugular vein engorgement. Concurrently, inferior vena cava also becomes dilated, causing dilation of the hepatic and portal veins, eventually resulting in hepatomegaly (congestive hepatopathy) with loss of appetite. Ascites and lower extremity edema may develop, which can elicit complaints of abdominal fullness. Furthermore, along with hepatomegaly, drainage from the splenic vein is impaired and splenomegaly is clinically manifested. Due to a decline of left ventricular preload and eventually of stroke volume, patients may experience fatigue and exertional dyspnea, and sometimes, indigestion and diarrhea. However, one thing that should be remembered is that severe TR may be present without these classical clinical signs or symptoms,26) and subjective symptoms may become evident only after irreversible organ damage occurs.4) Therefore, early referral (or timely referral) for corrective TR surgery, ideally before the development of heart failure symptoms, is of crucial importance (see next section), which highlights that serial echocardiography-based screening probably has a role to play in patients at high risk of TR development or in those with established severe TR. With regard to TR assessment, it should be kept in mind that, in contrast to mitral regurgitation, invasive right ventriculography is not helpful for assessing TR severity because the catheter may induce TR and sometimes hinder the accurate assessment of degree of TR.
As stated in previous sections, significant TR is not uncommon and it does develop more than infrequently in patients with no more than mild TR before index left side valve surgery.3)4) Although we have no data on the relative superiority of corrective TR surgery over optimal medical therapy, corrective TR surgery is expected to be advantageous in terms of prognosis if it is performed in a timely manner.4)7)27) In addition to improvements in symptoms and survival in severe TR patients, timely performed corrective TR surgery can induce RV reverse remodeling.7) However, the problem is that surgical results of corrective TR surgery have been reported to be poor, with an estimated early mortality rate range of 10% to 30%.4)28)29) According to available evidence, not TR surgery itself, but surrounding patient condition secondary to severe TR may be the culprit for determining operative outcome. A couple of years ago, our group published prospective data on the outcome of isolated TR surgery. In 61 patients with isolated severe TR prospectively enrolled, preoperative hemoglobin level, echocardiography-determined RV end-systolic area, and subjective symptoms emerged as the three important determinants of surgical outcomes in this population, although subjective symptoms did not achieve statistical significance.4) In another study performed in South Korea on patients with severe TR with or without combined left side valve disease, it was found that age, male gender, a NYHA class IV symptom, the presence of liver cirrhosis, preoperative hemoglobin level, preoperative albumin level, and estimated glomerular filtration rate were independent determinants of outcomes. Notably, neither procedural type nor TR etiology was found to be predictive of mortality. Taken together, despite the lack of a large amount of solid evidences, available evidence strongly suggests that corrective TR surgery can be performed with an acceptable operative risk if it is performed in a timely manner.4-6)28) Unfortunately, no appropriate guidelines for surgical timing in severe TR is available till now. Although the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) suggest guidelines regarding the operative timing for severe TR,26)30) these guidelines are not predicated on cumulative evidence. Practically, many recent studies have proposed that early surgery is of paramount importance in terms of improving postoperative short- and long-term outcomes. In particular, an earlier study suggested possibility that the restoration of normal life expectancy was not achieved even in patients with NYHA class II symptoms, highlighting the need for early surgical referral for corrective TR surgery before the development of heart failure symptoms.6) However, current ACC/AHA guidelines suggest tricuspid valve surgery for severe 'primary' TR only when symptom is present based on the level of evidence of C, and make no mention on isolated severe, secondary (functional) TR occurring in patients who have undergone prior left side valve surgery.26) More surprisingly, severe isolated, secondary (functional) TR with mild or no symptom is described as a contraindication for corrective surgery, which is in clear contrast to ESC guidelines in which corrective TR surgery can be recommended as class IIb indication.26)30) ESC guidelines are, to some extent, progressive and recommend corrective TR surgery in symptomatic patients with severe TR who had prior left side valve surgery, even in the absence of left side valve abnormalities.30) In many studies dealing with severe TR, surgical delay has been frequently recognized,4-7)16)17)28)31) and this is probably attributable, to some extent, to the absence of appropriate guidelines for surgical timing. Therefore, the establishment of guidelines based on widely used clinical, laboratory, and echocardiographic parameters is urgently required. Table 1 provides a side-by-side comparison of the current ACC/AHA vs. ESC guidelines for the surgical treatment of tricuspid valve disease.
In summary, based on current evidence, early surgical referral before the onset of subjective symptom and/or laboratory abnormalities (like anemia) and/or echocardiographic abnormalities is likely to render a better surgical outcome. Of these, subjective symptoms, hemoglobin level and echocardiographic index could be used to guide optimal surgical timing for severe TR.4)
NYHA functional class is the simplest and most frequently used parameter for assessing heart failure symptoms. Despite its simplicity, its prognostic value has been documented in the context of left side heart failure. The prognostic value of this unsophisticated index for predicting the surgical outcomes of patients with severe TR scheduled for corrective surgery remained uncertain until Kim et al.4) published this issue in Circulation in 2008. In this article, it was first suggested that NYHA functional class provides clues for optimal surgical timing. In the past, patients with severe TR were treated medically on a symptomatic basis using diuretics, digestives, and so on. This may have been due to a "fear" of referring patients with severe TR for surgery, because many clinicians believed that TR surgery was inevitably accompanied by high mortality and morbidity. With regard to surgery for severe mitral regurgitation, we no longer wait for the development of heart failure symptoms or overt left ventricular dysfunction before referral for corrective surgery, and this approach is now well accepted as a "standard-of-care" for patients with severe mitral regurgitation. However, we have not achieved any consensus in terms of optimal timing of corrective TR surgery. According to a small number of published articles,4-6) the current 'symptom-guided' surgical indication for severe TR almost always renders poor overall surgical outcomes. Instead, surgical correction of severe TR should be strongly considered before the development of heart failure symptoms. Kim et al.4) showed that, in patients with NYHA functional class II symptoms, operative mortality was only 4.8%, and that 2-year event-free survival rate was 90%, which was in clear contrast to the results obtained in patients with NYHA functional class III or IV symptoms. Another study also displayed that the presence of advanced heart failure symptoms is an independent determinant of mortality.5) Topilsky et al.6) showed in patients with severe TR with or without concomitant another valve surgery that operative mortality can be reduced to around 6% when patients are operated on before the development of NYHA functional class IV symptoms. More interestingly, they found that although mildly symptomatic patients (defined as those with NYHA class II or III symptoms) had obviously better surgical outcomes than patients with NYHA functional class IV symptoms, the restoration of normal life expectancy could not be attained even in patients with very mild symptoms (NYHA functional class II).6) This finding supports the concept that we need more sensitive surrogate markers for surgical outcomes in severe TR patients. We expect that laboratory and echocardiographic indexes could occupy important positions.
According to the current ACC/AHA and ESC guidelines,26)30) asymptomatic patients with severe mitral regurgitation should be referred to a cardiovascular surgeon for mitral valve repair or replacement when left ventricular systolic dysfunction, as represented by a decrease in left ventricular ejection fraction of ≤ 60% or an increase in left ventricular end-systolic dimension of ≥ 40 mm. These criteria suggest that indexes provided by echocardiography can more sensitively and reliably reflect disease progression. A similar strategy might be applicable to severe TR. However, in contrast to the left ventricle, the evaluation of RV function and size in a quantitative manner is challenging by two-dimensional echocardiography because of its complex geometry, as well as the limited definition of the endocardial surface attributable to heavy trabeculation of the RV myocardium.4)6) Notwithstanding, before the advent of three-dimensional echocardiography, RV end-systolic and end-diastolic area, and its derivative, RV fractional area change, were frequently used to estimate RV systolic function. As a matter of fact, in one study, RV end-systolic area emerged as an independent determinant of TR surgical outcome with a cutoff value of 20 cm2, which had a sensitivity of 73% and a specificity of 67% for the prediction of event-free survival.4) Assessment of early postoperative RV fractional area change can also provide valuable information additional to preoperative parameters in severe TR.28) However, these two-dimensional echocardiography indexes of the RV did not prove their ability in another study.6) This may have been because the measurement reproducibilities of these indexes are not as good as those of the left ventricle.32) The predominant orientation of muscle fibers in the RV is in the direction of the long axis, and based on this tricuspid annular systolic excursion and its velocity were introduced as potential indices reflecting RV systolic function. Since the first description of excellent correlation between RV systolic function and tricuspid annular plane systolic excursion,33) it has been used as an indirect index of RV systolic function. Actually, some authors found that it has a powerful ability to predict prognosis in pulmonary hypertension,34) mitral regurgitation,35) and heart failure.36) Besides, tissue Doppler technique-determined tricuspid annular systolic velocity has also been reported to have predictive value after corrective TR surgery.31) However, a recent publication reported a poor correlation between tricuspid annular plane systolic excursion and RV systolic function as determined by cardiac magnetic resonance imaging in patients with severe TR.37) Furthermore, we also demonstrated that tricuspid annular systolic velocity has limited accuracy for predicting the prognosis of patients with severe TR scheduled for being operated on in a larger study cohort.4)
As recently as 5 years ago, we did not have any reliable diagnostic imaging modality that could be routinely employed to assess the RV in our daily clinical practice, other than two-dimensional echocardiography. The lack of an appropriate method for effectively and quantitatively interrogating RV geometry and function may explain in part the small number of investigations conducted on the RV.24) However, we are now moving into a new epoch. Three-dimensional echocardiography and cardiac magnetic resonance imaging are two protagonists that can facilitate routine RV evaluations. In fact, these two diagnostic imaging modalities are now being incorporated into our daily clinical routine. Min et al.25) clearly illustrated the value of real-time three-dimensional echocardiography in terms of identifying patients at high risk for severe residual TR after TR surgery. With cardiac magnetic resonance imaging, we found that successful TR surgery can lead to a significant rise in the left ventricular preload and cardiac index, which subsequently contribute to a significant amelioration of the functional capacity of the TR patients.7) In addition, we suggested that cardiac magnetic resonance imaging could be helpful for determining the optimal timing for TR surgery.7) Although these two imaging modalities are still in their infancies, they may be of tremendous practical use for the assessment of RV systolic function in relation to right side myocardial or valvular diseases in near future.
Earlier reports continue to mention the close relationship between anemia and prognosis in left side heart failure.38-41) Moreover, it has been strongly suggested that iron depletion is a main culprit for anemia in many cases of heart failure,42)43) and that its supplementation can significantly improves prognosis in these patients.44)45) Low renal perfusion, malabsorption, nutritional deficiencies, and hemodilution have also been proposed as potential mechanisms responsible for anemia observed in patients with left side heart failure, and these mechanisms may be involved in the development of anemia in patients with severe TR. However, hypersplenism secondary to long-standing systemic venous congestion resulting from chronic volume overload to the RV, right atrium, and subsequently portal vein and splenic vein is strongly suggested as the main mechanism underlying the development of anemia.4) This is further advocated by concomitant manifestations of low platelet count and hypoalbuminemia in many patients with severe TR.4)5)28) In this regard, hemoglobin levels in patients with severe TR should indicate the chronicity of systemic venous congestion, and thus, can be objectively mirror disease severity in these patients. Such relations indicate that approaches to severe TR and combined anemia should be dealt with in different ways. In our previous study,4) we suggested preoperative hemoglobin cutoff of 11.3 g/dL for predicting event-free survival in severe TR. According to the WHO definition, a hemoglobin level of < 13 g/dL for men or of < 12 g/dL for women defines the presence of anemia. Surprisingly, the hemoglobin cutoff value we previously suggested is very close to 12 g/dL for women.4) Considering that the majority of the patients enrolled in this study were women,4) the cutoff value for men used for determining optimal surgical timing for severe TR is likely to be slightly higher, that is, around 13 g/dL. This issue requires further investigations in more detail in near future. Nevertheless, based on currently available evidences, in order to improve surgical outcome, early referral for corrective TR surgery appears desirable before the preoperative hemoglobin level reaches about 11 g/dL.
Brain natriuretic peptide (BNP) is primarily secreted by the right or left ventricles in response to ventricular volume and/or pressure overload.46) Plasma BNP levels have been shown to rise in patients with heart failure and ventricular hypertrophy.47)48) Similar phenomena could be observed in patients with left side valvular diseases like aortic stenosis and mitral regurgitation.49-51) In particular, elevated BNP levels were found to be significantly correlated with poor functional capacity and dismal prognosis.49-51) This may also be true in patients with severe TR who undergo corrective surgery. Despite a limited number of patients, we found that an elevation in BNP level in patients with isolated severe TR probably predicts adverse surgical outcomes after corrective TR surgery.52) This issue should be confirmed in larger TR cohorts.
Thanks to the significant progress made in surgical techniques and supportive medical care, left side valve surgeries now achieve operative mortality rates as low as 1-2% in experienced cardiac centers.53) Unfortunately, the results of corrective TR surgery are yet disappointing in comparison with fascinating results achieved in the field of left side valve surgeries. This is not altogether surprising, because until recently TR was regarded as a relatively unimportant 'phenomenon', and not as an important 'disease'. Thus, in the recent past, surgical timings have been delayed in many TR patients, resulting in residual clinical comorbidities like cardiac cirrhosis and atrial fibrillation in many cases. Therefore, even after successful corrective TR surgery, patients should be carefully followed for possible remaining comorbidities that could substantially diminish quality of life. Furthermore, optimal medical therapy should be continued without interruption with the help of loop diuretics, spironolactone, and warfarin. Despite a lack of relevant data regarding this issue, careful and total care should be exercised before and after corrective TR surgery.
Significant TR should not be ignored because it substantially affects quality of life, exercise capacity, and survival. Given that successful left side valve surgery is not always accompanied by the elimination of functional TR, concomitant TR correction (even in mild degree of TR) and/or the maze operation (in the setting of atrial fibrillation) should be strongly considered at the time of left side valve surgery. Although the determination of optimal surgical timing is the most important issue under active investigation, timely surgical correction of isolated TR can significantly improve RV volume and function, cardiac index, and exercise capacity. Due to the complex geometry of the RV, two-dimensional assessments are inherently limited. Accordingly, researches regarding diagnostic and prognostic value of three-dimensional echocardiography and cardiac magnetic resonance imaging are warranted in future. Because many patients that successfully survive corrective TR surgery tend to suffer from comorbidities, further studies are required to determine optimal medication and care strategies for these patients.
Figures and Tables
Fig. 1
Event-free cumulative survival rates according to the presence or absence of late development of significant tricuspid regurgitation (TR). Note that survival curve begins to diverge approximately 130 months after left-sided valve surgery, highlighting that more than 10 years of careful clinical and echocardiographic follow-up is clearly indicated to evaluate whether or not significant TR is developed after left-sided valve surgery. Reprinted, with permission, from Am Heart J 2008;155:732-7.3)

Fig. 2
On preoperative two-dimensional (A) and Doppler (B) echocardiography, there was no doubt that severe tricuspid regurgitation (TR) is present in this woman. The problem was that even after tricuspid annuloplasty with an annular ring, severe TR was still there and was not gone (C and D). In this example, we can understand that mechanisms other than tricuspid annular dilation may be present in accounting for development of functional TR (A and B: Preoperative. C and D: Postoperative).
Acknowledgements
This study was partly supported by grants from Chong Kun Dang Pharm Research fund 2012.
References
1. Nihoyannopoulos P. Tricuspid valvectomy following tricuspid valve endocarditis on an intravenous drug addict. Heart. 2001. 86:144.

2. Kim HK, Kim YJ, Kim KI, Jo SH, Kim KB, Ahn H, Sohn DW, Oh BH, Lee MM, Park YB, Choi YS. Impact of the maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005. 112:9 Suppl. I14–I19.

3. Kwak JJ, Kim YJ, Kim MK, Kim HK, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008. 155:732–737.

4. Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, Park YB. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009. 120:1672–1678.

5. Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Surgical outcomes of severe tricuspid regurgitation: predictors of adverse clinical outcomes. Heart. 2013. 99:181–187.

6. Topilsky Y, Khanna AD, Oh JK, Nishimura RA, Enriquez-Sarano M, Jeon YB, Sundt TM, Schaff HV, Park SJ. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011. 123:1929–1939.

7. Kim HK, Kim YJ, Park EA, Bae JS, Lee W, Kim KH, Kim KB, Sohn DW, Ahn H, Park JH, Park YB. Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J. 2010. 31:1520–1528.

8. Groves PH, Lewis NP, Ikram S, Maire R, Hall RJ. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J. 1991. 66:295–301.

9. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004. 43:405–409.

10. Czer LS, Maurer G, Bolger A, DeRobertis M, Kleinman J, Gray RJ, Chaux A, Matloff JM. Tricuspid valve repair. Operative and follow-up evaluation by Doppler color flow mapping. J Thorac Cardiovasc Surg. 1989. 98:101–110. discussion 110-1.
11. Goldman ME, Guarino T, Fuster V, Mindich B. The necessity for tricuspid valve repair can be determined intraoperatively by two-dimensional echocardiography. J Thorac Cardiovasc Surg. 1987. 94:542–550.

12. Hannoush H, Fawzy ME, Stefadouros M, Moursi M, Chaudhary MA, Dunn B. Regression of significant tricuspid regurgitation after mitral balloon valvotomy for severe mitral stenosis. Am Heart J. 2004. 148:865–870.

13. McGrath LB, Gonzalez-Lavin L, Bailey BM, Grunkemeier GL, Fernandez J, Laub GW. Tricuspid valve operations in 530 patients. Twenty-five-year assessment of early and late phase events. J Thorac Cardiovasc Surg. 1990. 99:124–133.
14. Porter A, Shapira Y, Wurzel M, Sulkes J, Vaturi M, Adler Y, Sahar G, Sagie A. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999. 8:57–62.
15. Je HG, Song H, Jung SH, Choo SJ, Song JM, Kang DH, Yun SC, Chung CH, Song JK, Lee JW. Impact of the Maze operation on the progression of mild functional tricuspid regurgitation. J Thorac Cardiovasc Surg. 2008. 136:1187–1192.

16. Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsuo T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg. 2003. 75:1826–1828.

17. Izumi C, Iga K, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis. 2002. 11:353–356.
18. Stulak JM, Schaff HV, Dearani JA, Orszulak TA, Daly RC, Sundt TM 3rd. Restoration of sinus rhythm by the Maze procedure halts progression of tricuspid regurgitation after mitral surgery. Ann Thorac Surg. 2008. 86:40–44. discussion 44-5.

19. Ubago JL, Figueroa A, Ochoteco A, Colman T, Duran RM, Duran CG. Analysis of the amount of tricuspid valve anular dilatation required to produce functional tricuspid regurgitation. Am J Cardiol. 1983. 52:155–158.

20. Mikami T, Kudo T, Sakurai N, Sakamoto S, Tanabe Y, Yasuda H. Mechanisms for development of functional tricuspid regurgitation determined by pulsed Doppler and two-dimensional echocardiography. Am J Cardiol. 1984. 53:160–163.

21. Come PC, Riley MF. Tricuspid anular dilatation and failure of tricuspid leaflet coaptation in tricuspid regurgitation. Am J Cardiol. 1985. 55:599–601.

22. Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, Thomas JD, Shiota T. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005. 111:975–979.

23. Fukuda S, Gillinov AM, McCarthy PM, Stewart WJ, Song JM, Kihara T, Daimon M, Shin MS, Thomas JD, Shiota T. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2006. 114:1 Suppl. I582–I587.

24. Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB, Choi YS. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006. 98:236–242.

25. Min SY, Song JM, Kim JH, Jang MK, Kim YJ, Song H, Kim DH, Lee JW, Kang DH, Song JK. Geometric changes after tricuspid annuloplasty and predictors of residual tricuspid regurgitation: a real-time three-dimensional echocardiography study. Eur Heart J. 2010. 31:2871–2880.

26. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. 2006 Writing Committee Members. American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008. 118:e523–e661.
27. Yang WI, Shim CY, Kang MK, Chang HJ, Chung N, Cho SY, Ha JW. Vena contracta width as a predictor of adverse outcomes in patients with severe isolated tricuspid regurgitation. J Am Soc Echocardiogr. 2011. 24:1013–1019.

28. Park K, Kim HK, Kim YJ, Cho GY, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, Park YB. Incremental prognostic value of early postoperative right ventricular systolic function in patients undergoing surgery for isolated severe tricuspid regurgitation. Heart. 2011. 97:1319–1325.

29. Filsoufi F, Anyanwu AC, Salzberg SP, Frankel T, Cohn LH, Adams DH. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg. 2005. 80:845–850.

30. Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology. ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007. 28:230–268.

31. Kwon DA, Park JS, Chang HJ, Kim YJ, Sohn DW, Kim KB, Ahn H, Oh BH, Park YB, Choi YS. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol. 2006. 98:659–661.

32. Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Vliegen HW, Delgado V. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2012. 5:628–636.

33. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984. 107:526–531.

34. Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006. 174:1034–1041.

35. Dini FL, Fontanive P, Panicucci E, Andreini D, Chella P, De Tommasi SM. Prognostic significance of tricuspid annular motion and plasma NT-proBNP in patients with heart failure and moderate-to-severe functional mitral regurgitation. Eur J Heart Fail. 2008. 10:573–580.

36. Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Randé JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012. 18:216–225.

37. Hsiao SH, Lin SK, Wang WC, Yang SH, Gin PL, Liu CP. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr. 2006. 19:902–910.

38. Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, Wahi PL, Poole-Wilson PA, Harris P. Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation. 1992. 86:12–21.

39. Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, Chiang YT, Aknay N, Maggioni AP, Opasich C, Latini R, Cohn JN. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation. 2005. 112:1121–1127.

40. Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006. 113:2713–2723.

41. Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, Shin MS, Seong IW, Ahn Y, Kang SM, Kim YJ, Kim HS, Chae SC, Oh BH, Lee MM, Ryu KH. KorHF Registry. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the korean heart failure registry. Korean Circ J. 2011. 41:363–371.

42. Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011. 58:474–480.

43. Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011. 58:1241–1251.
44. Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J. 2013. 34:30–38.

45. Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012. 14:423–429.

46. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991. 87:1402–1412.

47. Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T. Combined neutral endopeptidase and angiotensin-converting enzyme inhibition in heart failure: role of natriuretic peptides and angiotensin II. J Cardiovasc Pharmacol. 1998. 31:116–125.

48. Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008. 117:3010–3019.
49. Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC Jr, Enriquez-Sarano M. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005. 111:2391–2397.

50. Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, Krauss JG, Cagide AM. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009. 54:1099–1106.

51. Hwang IC, Kim YJ, Kim KH, Lee SP, Kim HK, Sohn DW, Oh BH, Park YB. Prognostic value of B-type natriuretic peptide in patients with chronic mitral regurgitation undergoing surgery: mid-term follow-up results. Eur J Cardiothorac Surg. 2013. 43:e1–e6.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download