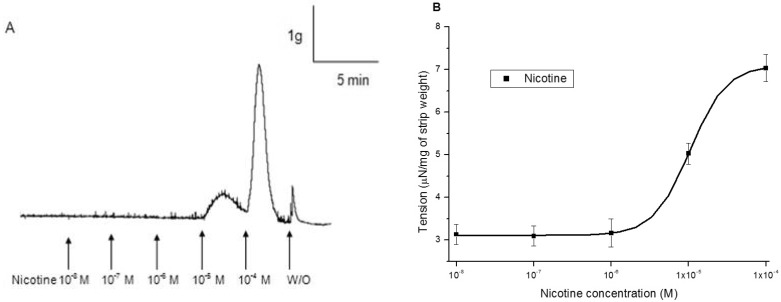
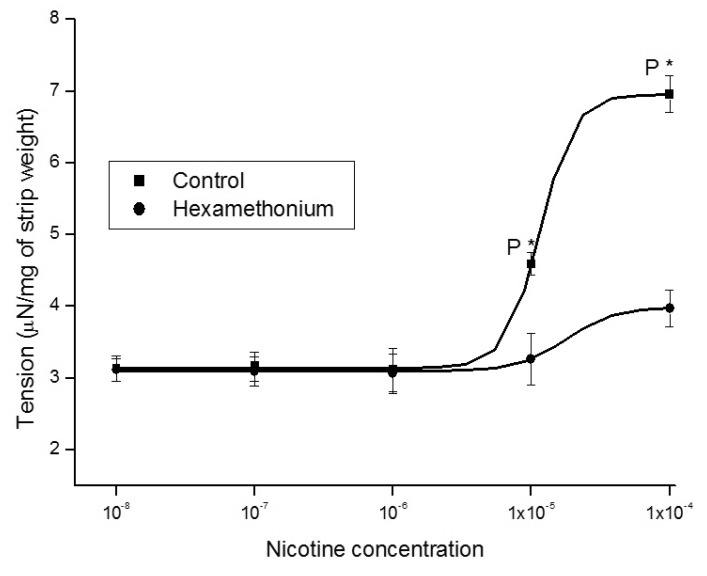
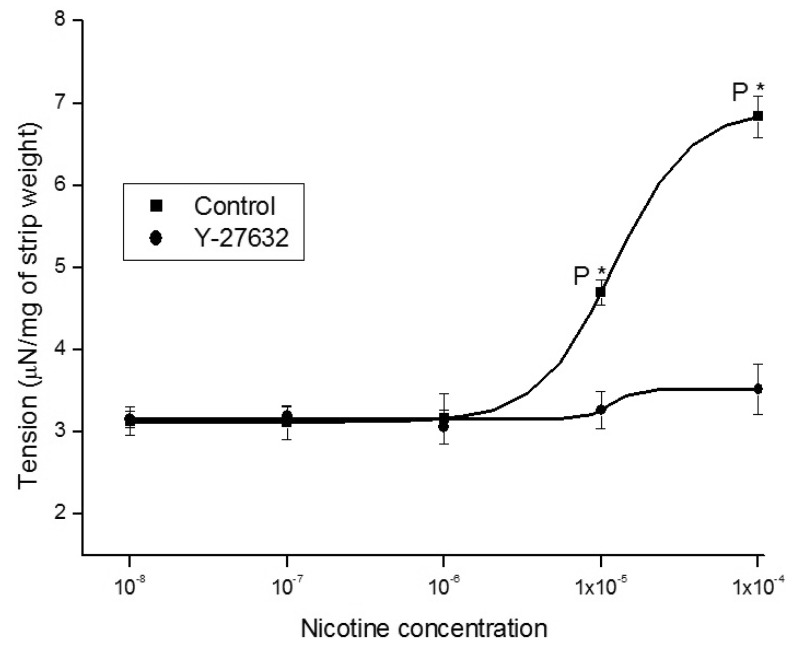
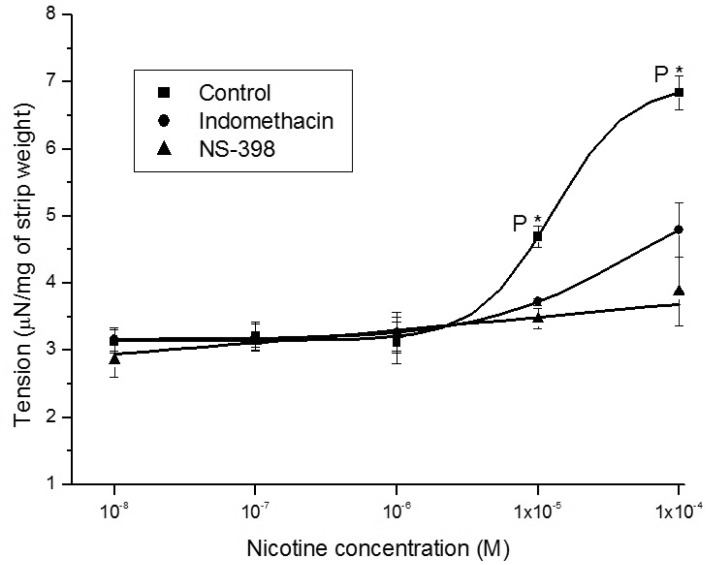
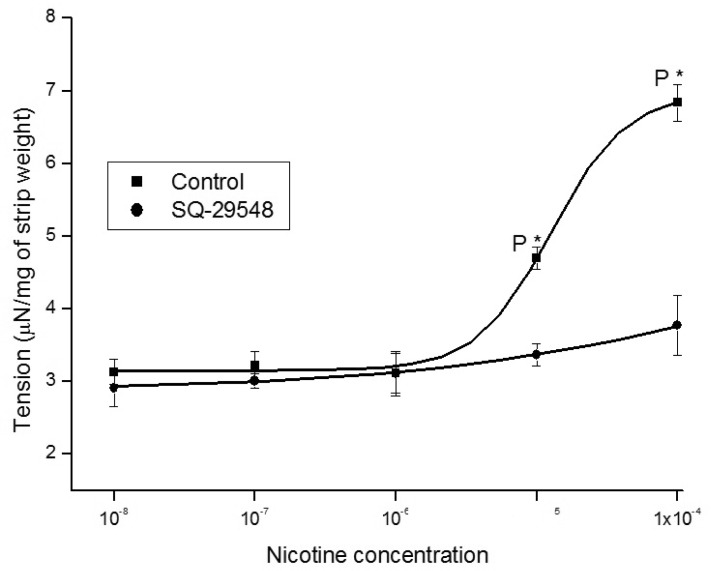
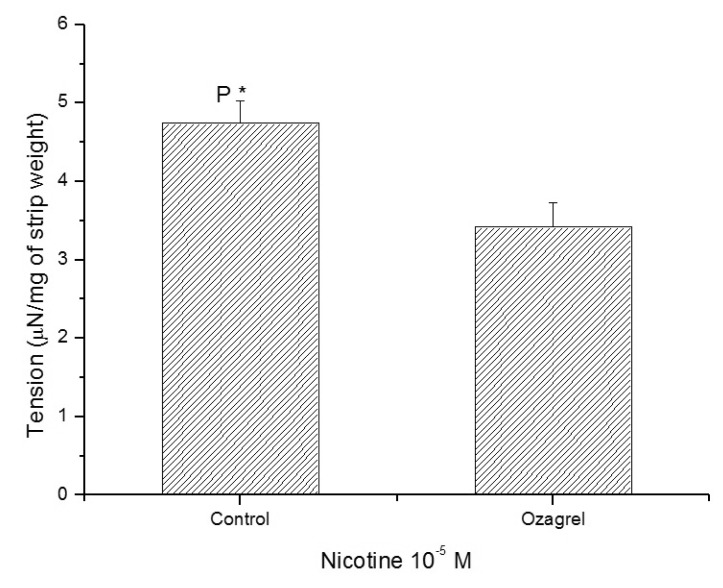
1. McVary KT, Carrier S, Wessells H. Subcommittee on Smoking and Erectile Dysfunction Socioeconomic Committee. Sexual Medicine Society of North America. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001; 166:1624–1632. PMID:
11586190.

2. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–1030. PMID:
2927481.

3. Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005; 55:281–299. PMID:
16166074.

4. Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57:79–115. PMID:
15734728.

5. Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996; 36:597–613. PMID:
8725403.

6. Zainalabidin S, Budin SB, Ramalingam A, Lim YC. Aortic remodelling in chronic nicotine-administered rat. Korean J Physiol Pharmacol. 2014; 18:411–418. PMID:
25352761.

7. Pickering TG, Schwartz JE, James GD. Ambulatory blood pressure monitoring for evaluating the relationships between lifestyle, hypertension and cardiovascular risk. Clin Exp Pharmacol Physiol. 1995; 22:226–231. PMID:
7554420.

8. Gunby P. Surgeon General emphasizes nicotine addiction in annual report on tobacco use, consequences. JAMA. 1988; 259:2811. PMID:
3367442.

9. Hanna ST. Nicotine effect on cardiovascular system and ion channels. J Cardiovasc Pharmacol. 2006; 47:348–358. PMID:
16633075.
10. Hayashida H, Okamura T, Tomoyoshi T, Toda N. Neurogenic nitric oxide mediates relaxation of canine corpus cavernosum. J Urol. 1996; 155:1122–1127. PMID:
8583577.

11. Bagcivan I, Gokce G, Yildirim S, Sarioglu Y. Investigation of the mechanism of nicotine induced relaxation in rabbit corpus cavernosum in vitro. Urol Res. 2004; 32:209–212. PMID:
15205855.

12. Klinge E, Alaranta S, Sjöstrand NO. Pharmacological analysis of nicotinic relaxation of bovine retractor penis muscle. J Pharmacol Exp Ther. 1988; 245:280–286. PMID:
3361446.
13. Bozkurt NB, Vural IM, Sarioglu Y, Pekiner C. Nicotine potentiates the nitrergic relaxation responses of rabbit corpus cavernosum tissue via nicotinic acetylcholine receptors. Eur J Pharmacol. 2007; 558:172–178. PMID:
17208220.

14. Hayashida H, Fujimoto H, Yoshida K, Tomoyoshi T, Okamura T, Toda N. Comparison of neurogenic contraction and relaxation in canine corpus cavernosum and penile artery and vein. Jpn J Pharmacol. 1996; 72:231–240. PMID:
8957684.

15. Ozturk Fincan GS, Vural IM, Ercan ZS, Sarioglu Y. Enhancement effects of nicotine on neurogenic relaxation responses in the corpus cavernosum in rabbits: the role of nicotinic acetylcholine receptor subtypes. Eur J Pharmacol. 2010; 627:281–284. PMID:
19878666.

16. Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009; 89:73–120. PMID:
19126755.

17. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003; 27:201–206. PMID:
14627618.

18. Rees RW, Ralph DJ, Royle M, Moncada S, Cellek S. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol. 2001; 133:455–458. PMID:
11399661.

19. Wingard CJ, Husain S, Williams J, James S. RhoA-Rho kinase mediates synergistic ET-1 and phenylephrine contraction of rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R1145–R1152. PMID:
12893655.

20. Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976; 261:558–560. PMID:
934294.

21. Clària J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003; 9:2177–2190. PMID:
14529398.
22. Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, van de. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000; 43:4–13. PMID:
10643694.

23. Ji X, Nishihashi T, Trandafir CC, Wang A, Shimizu Y, Kurahashi K. Pharmacological nature of nicotine-induced contraction in the rat basilar artery: involvement of arachidonic acid metabolites. Eur J Pharmacol. 2007; 577:109–114. PMID:
17765890.

24. Daley JT, Brown ML, Watkins T, Traish AM, Huang YH, Moreland RB, De Tejada IS. Prostanoid production in rabbit corpus cavernosum: I. regulation by oxygen tension. J Urol. 1996; 155:1482–1487. PMID:
8632615.

25. Daley JT, Watkins MT, Brown ML, Martinez V, Cuevas P, Saenz de. Prostanoid production in rabbit corpus cavernosum. II. Inhibition by oxidative stress. J Urol. 1996; 156:1169–1173. PMID:
8709340.
26. Moreland RB, Albadawi H, Bratton C, Patton G, Goldstein I, Traish A, Watkins MT. O2-dependent prostanoid synthesis activates functional PGE receptors on corpus cavernosum smooth muscle. Am J Physiol Heart Circ Physiol. 2001; 281:H552–H558. PMID:
11454556.

27. Angulo J, Cuevas P, La Fuente JM, Pomerol JM, Ruiz-Castañé E, Puigvert A, Gabancho S, Fernández A, Ney P, Sáenz De. Regulation of human penile smooth muscle tone by prostanoid receptors. Br J Pharmacol. 2002; 136:23–30. PMID:
11976264.
28. Hedlund H, Andersson KE. Contraction and relaxation induced by some prostanoids in isolated human penile erectile tissue and cavernous artery. J Urol. 1985; 134:1245–1250. PMID:
3903226.