Abstract
Endoplasmic reticulum (ER) stress, unfolded protein response (UPR), and mitochondrial biogenesis were assessed following varying intensities of exercise training. The animals were randomly assigned to receive either low- (LIT, n=7) or high intensity training (HIT, n=7), or were assigned to a control group (n=7). Over 5 weeks, the animals in the LIT were exercised on a treadmill with a 10° incline for 60 min at a speed of 20 m/min group, and in the HIT group at a speed of 34 m/min for 5 days a week. No statistically significant differences were found in the body weight, plasma triglyceride, and total cholesterol levels across the three groups, but fasting glucose and insulin levels were significantly lower in the exercise-trained groups. Additionally, no statistically significant differences were observed in the levels of PERK phosphorylation in skeletal muscles between the three groups. However, compared to the control and LIT groups, the level of BiP was lower in the HIT group. Compared to the control group, the levels of ATF4 in skeletal muscles and CHOP were significantly lower in the HIT group. The HIT group also showed increased PGC-1α mRNA expression in comparison with the control group. Furthermore, both of the trained groups showed higher levels of mitochondrial UCP3 than the control group. In summary, we found that a 5-week high-intensity exercise training routine resulted in increased mitochondrial biogenesis and decreased ER stress and apoptotic signaling in the skeletal muscle tissue of rats.
The endoplasmic reticulum (ER) has an important role in protein synthesis, protein maturation, and calcium homeostasis. Biological changes due to energy deprivation and calcium depletion may cause severe ER stress, resulting in the activation of the unfolded protein response (UPR) [1]. UPR triggers an increase in the expression of chaperones that guide protein folding; however, if such a response fails to restore homeostatic balance, apoptotic signaling may occur [2]. Chronic ER stress has been found to be involved in many diseases including fibrosis, atherosclerosis, diabetes, and neurodegenerative diseases [3,4,5,6,7]. An increase in ER stress has been reported in the adipose tissue and liver of diet-induced obese mice and ob/ob mice [8], and islet cells from mice and humans with type 2 diabetes have shown increased ER stress [9]. Biomarkers of the UPR were found to be increased by palmitate in isolated myocytes from diabetic patients, indicating ER stress in the skeletal muscle of diabetic patients in vivo [6].
Exercise is one of the therapies for the treatment of obesity and type 2 diabetes [10], and the known benefits of regular exercise on skeletal muscles include increased levels of glucose transporters [11], antioxidants [12], and fatty acid oxidation enzymes [13]. Muscle contraction during exercise results in the production of reactive oxygen species (ROS) and a subsequent increase in the expression of antioxidant enzymes [14]. This increase of ROS may cause ER stress, resulting in an increase in the production of cellular antioxidants to protect against cell damage. While the relationship between mitochondrial dysfunction and ER stress has been investigated in skeletal muscle tissue of high-fat fed rats in a recent report [15], there have been few studies investigating ER stress in relation to exercise and mitochondrial activity, despite its potential influence on metabolism throughout the whole body.
Recently, several studies have investigated ER stress occurring in muscle tissue during exercise [16,17]. Activation of UPR was observed in human skeletal muscle after a 200-km race [17], and a 4-week period of exercise training resulted in increased UPR as an adaptation to ER stress [16]. Thus, implying that exercise training may induce an ER-stress adaptation in skeletal muscle, and it reported that muscle contraction is directly involved in the activation of the UPR. In addition, mitochondrial biogenesis was also demonstrated to have a key role in controlling UPR [16]. Skeletal muscles require a large amount of energy; as a result, mitochondria play a more important role in skeletal muscle than in other tissues. Therefore, the relationship between mitochondria and ER stress in skeletal muscle was suggested as an area for further study [18]. In addition, the therapeutic effects of exercise and the biological responses it induces are known to be closely related to the intensity of exercise [19]. Moreover, these biological responses vary according to exercise intensity and therefore induce different degrees of ER stress. Thus, we decided to compare ER stress, apoptosis signaling, and mitochondrial biogenesis in low- and high-intensity exercise-trained rats.
To this end, following exercise training in rats, we examined the changes in the level of immunoglobulin heavy chain-binding protein (BiP) in order to detect any disturbances in ER proteins and in the protein kinase RNA-like ER kinase (PERK)-activating transcription factor (ATF) 3/4-C/EBP-homologous protein (CHOP) pathway to assess adaptations to exercise. In addition, we examined changes in the levels of peroxisome proliferator-activator receptor gamma coactivator-1 alpha (PGC-1α) and uncoupling protein 3 (UCP-3) in order to understand the relationship between adaptations to ER stress and mitochondrial-biogenesis gene expression.
Male Sprague-Dawley rats, each weighing approximately 250 g, were purchased from Samtako (Seoul, Korea). The animals were acclimatized for 1 week prior to beginning the experiment by exposure to 12-hour light/dark cycles. The animals treated in accordance with the principles of the Guide to the Care and Use of Experimental Animals of the Yeungnam University Medical Center. The animals were assigned randomly to one of three experimental groups; control (n=7), low-intensity training (LIT, n=7), or high-intensity training (HIT, n=7). Before beginning the exercise protocols, the rats of both exercise groups were adapted to running on a treadmill for 15 min, at increasing speeds from 0~15 m/min, once per day for 5 days. After this adaptation, the rats ran on treadmills with a 10° incline for a period of 60 min on five days per study week. The treadmill speeds were set at 20 m/min in the LIT group and 34 m/min in the HIT group. This experiment was performed as described previously [20]. The trained rats were sacrificed 3 days after the final exercise session to exclude the possible effects of acute exercise stress in the analysis.
The rats were anesthetized with intra-peritoneal injections of 20 mg/kg tiletamine and zolazepam (Zoletil®; Virbac, Carros, France), and 10 mg/kg of xylazine hydrochloride (Rompun®, Bayer, Monheim, Germany). Food was removed from the cages before sacrifice. After complete anesthesia, the abdominal cavity was opened rapidly following the median line of the abdomen. Blood was drawn rapidly from the abdominal vena cava into syringes. Blood was centrifuged (1800×g for 10 min, 4℃) and the plasma was maintained at -80℃ for the measurement of triacylglycerol (TAG), total cholesterol (TC), glucose, and insulin levels. The gastrocnemius muscle was collected immediately from each rat, and was frozen and stored at -80℃ until the analytical assay was performed. Plasma TAG, TC, and glucose levels were measured using an enzymatic colorimetric assay kit from Sigma-Aldrich (St. Louis, Missouri, USA). Plasma insulin was measured using an enzyme-linked immunosorbent assay kit from Millipore (Billerica, MA, USA).
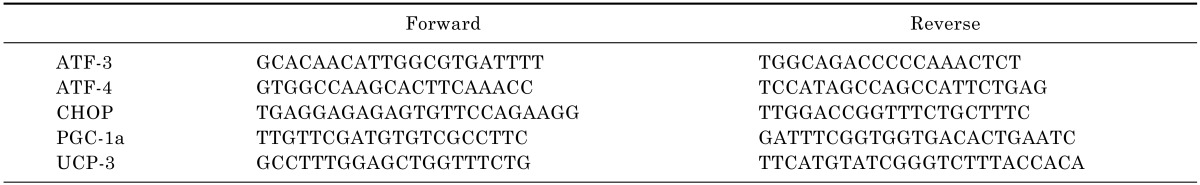
Frozen tissue samples were homogenized using an Ultra-Turrax T25 (IKA-Labortechnik, Staufel, Germany), and approximately 100 mg of tissue was used for the isolation of RNA. Total RNA was extracted using the guanidine thiocyanate method, and mRNA was purified using a PureLink RNA Mini Kit (Invitrogen, New York, USA) according to the manufacturer's instructions. Total RNA was reverse transcribed in a final volume of 20 µL using a High-Capacity cDNA Reverse Transcription Kit with random primers (Applied Biosystems, Foster City, California, USA) according to the manufacturer's instructions. Reverse-transcribed samples were stored at -20℃. The RNA level during PCR was measured with a continuous fluorescence detector using the Real-Time PCR 7500 system (MJ Research, Waltham, MA) and Power SYBR Green PCR master mix (Applied Biosystems, Foster City, California, USA) according the manufacturers' instructions. To normalize the amount of total RNA present in each reaction, the GAPDH gene was amplified simultaneously. Primers were designed using Primer Express software (Applied Biosystems) and are shown in Table 1. RT-PCR reactions were performed according to the following cycle profile: 10 min at 95℃, 44 cycles for 30 sec at 94℃, 1 min at 58℃, and 2 min at 68℃.
The gastrocnemius muscle was homogenized in lysis buffer containing 50 mM HEPES, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamide, 1 mM Na3VO4, 1 mM dithiothreitol (DTT), 5 mM MgCl2, 1% NP40, 10% glycerol, aprotinin, leupeptin, and pepstatin A. The homogenate was centrifuged at 20000×g for 15 min at 4℃. The protein concentration of the supernatant was measured using the Bradford assay. SDS-PAGE was used to separate 80 µg of protein that was then blotted on a PVDF membrane. The membrane was blocked with 0.1% Tween in tris-buffered saline (TBST) with 5% skimmed milk for 1 h at room temperature. Primary antibodies (PERK, phosphorylated PERK, BiP, CHOP, and GAPDH [PERK, phosphorylated PERK, BiP, and CHOP from Cell Signaling, Danvers, MA, USA; GAPDH from Santa Cruz Biotechnology, Santa Cruz, CA, USA]) were diluted 1:700 in TBST with 5% bovine serum albumin, and were incubated overnight at 4℃. The membranes were incubated with goat anti-rabbit/mouse IgG HRP antibodies (Bio-Rad, Hercules, CA, USA) for 1 h at room temperature then diluted 1:2000 in TBST with 5% skim milk. The membrane was examined using electrochemiluminescence. In this study, GAPDH was used as a control to ensure equal protein loading on the gel and the blots were quantified using MultiGauge software.
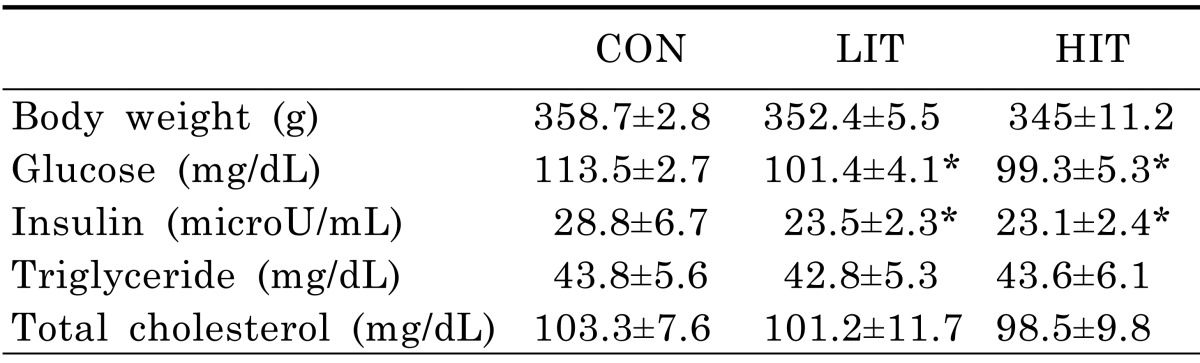
Although the average body weights of mice in the LIT (352.4±5.5 g) and HIT (345±11.2 g) groups were lower than in the untrained control group (358.7±2.8 g) after 5 weeks of training, there was no statistically significant difference among the groups (Table 2). The fasting glucose levels of the LIT (101.4±4.1 mg/dL) and HIT (99.3±5.3 mg/dL) groups following 5 weeks were lower than the control group (113.5±2.7 mg/dL). In addition, fasting insulin levels in mice from the LIT (23.5±2.3 µU/mL) and HIT (23.1±2.4 µU/mL) groups were lower than the control group (28.8±6.7 µU/mL). However, there was no significant difference between LIT and HIT groups in terms of fasting glucose and insulin levels. The TAG and TC levels in the fasting state did not show any statistically significant differences across all three groups.
To understand the effects of training intensity on ER stress and UPR in skeletal muscle, we measured PERK phosphorylation, BiP levels and ATF3/4 mRNA expression after 5 weeks of training. Although both intensities of training induced marginal increases in phosphorylation of PERK in skeletal muscle, there were no significant differences across the three groups (Fig. 1A). On the other hand, the HIT group showed significantly reduced BiP levels (32.5%) in skeletal muscle tissue in comparison with the control group (Fig. 1B). LIT-group rats exhibited higher ATF3 gene expression (44.8%) in skeletal muscle tissue than the control group; however, the HIT group did not show any significant difference. Gene expression for ATF4 in the skeletal muscle tissue was significantly decreased in the HIT group (33.0%) in comparison with the control group (Fig. 1C). However, there was no difference in ATF4 gene expression between the control and LIT groups.
Conditions ranging from unsuccessful UPR to chronic ER stress can induce apoptosis; therefore, changes in the apoptotic pathway were examined using the levels of CHOP protein and CHOP mRNA. We found a 51.4% decrease in CHOP gene expression in the HIT group in comparison with the control group (Fig. 2A). The level of CHOP protein was somewhat decreased in the HIT group but the difference between the groups was not significant (Fig. 2B).
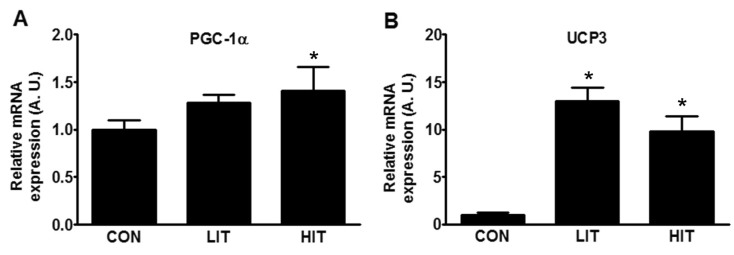
Owing to certain differences in UPR that were dependent on the intensity of exercise training, we attempted to assess the physiological changes in skeletal muscle during training. Mitochondrial biogenesis was measured by the expression levels of the proteins PGC-1α, and UCP3. An increase in the mRNA expression of PGC-1α of 41.5% was found in the HIT group in comparison with the control group (Fig. 3A). The expression of UCP3 in both the trained groups increased; it demonstrated approximately 13-fold (LIT) and 10-fold (HIT) increases in comparison with the control group (Fig. 3B).
We observed ER stress, apoptosis signaling, and mitochondrial biogenesis in the skeletal muscle of rats following LIT and HIT to clarify the adaptation mechanisms in muscle physiology induced by training. The duration of training (5 weeks) was selected in order to avoid focusing on the metabolic benefits associated with long-term training, such as with a 12-week training program [20,21]; this study focused on the effects of exercise training on skeletal muscles. The 5-week duration is similar to that of recent studies investigating ER stress and exercise training [16,22]. In previous studies, ER stress markers in the skeletal muscle in ob/ob mice and high-fat fed mice were unchanged by training [8], and 2 weeks of muscle unloading in the skeletal muscles of rats also did not induce UPR [23]. However, more recent studies demonstrated definite UPR in the skeletal muscle of mice [16] and humans [17]. After a 200-km run, levels of BiP, ATF4 and XBP1 mRNA increased in comparison with basal level [17]. These results demonstrated a definite role for the UPR in skeletal muscle physiology during short-term exercise and training.
There are three main effectors of ER stress: ATF6, inositol-requiring enzyme 1 alpha, and PERK [1]. Activated PERK, a type I ER transmembrane protein kinase, inhibits general protein translation in cells through the inactivation of the initiation factor eIF2a [24]; prolonged activation of PERK can result in either cellular adaptation to ER stress or apoptosis [25]. Our results from muscles after 5 weeks of training did not show any statistically significant increase in PERK phosphorylation, suggesting the adaptation of muscle cells to ER stress by repeated exercise; in addition, there was no indication of significant ER stress at the time of sacrifice. Therefore, the observed UPRs can be considered to be the result of adaption to exercise training and not the result of acute exercise. The Hsp70 ER chaperone, BiP, is considered an indicator of disturbances in ER protein homeostasis, and the accumulation of unfolded/misfolded proteins can result in the release of BiP from three effectors in order to transmit signals of ER stress [26,27]. BiP was found to be increased in the skeletal muscles of humans after a long distance run [17] but there were no significant changes in BiP levels in the skeletal muscles of mice following 4 weeks of training [16]. BiP levels in the LIT group in our study did not differ from those in the control group. However, BiP levels in the HIT group were significantly decreased in comparison with the control and LIT groups. This implies that the adaptive response was greater, resulting in decreased ER stress in skeletal muscle, following high-intensity exercise training.
In this study, HIT repressed the expression of ATF4 in comparison with the control and LIT groups. This translates to decreased ER stress in skeletal muscle following 5 weeks of HIT. It is known that during UPR, ATF4 upregulates genes that are involved in amino acid uptake, glutathione biosynthesis, and resistance to oxidative stress [28]. In a previous study, the gene expression of ATF4 was increased after a single exercise session, but ATF4 expression was decreased after 4 weeks of exercise training [16]. We cannot explain the reason for the difference in ATF3 gene expression between the LIT and HIT groups; LIT increased ATF3 in comparison with the control and HIT groups. However, this result implies that the ATF3 and ATF4 may be involved in different pathways leading to adaptation to exercise training.
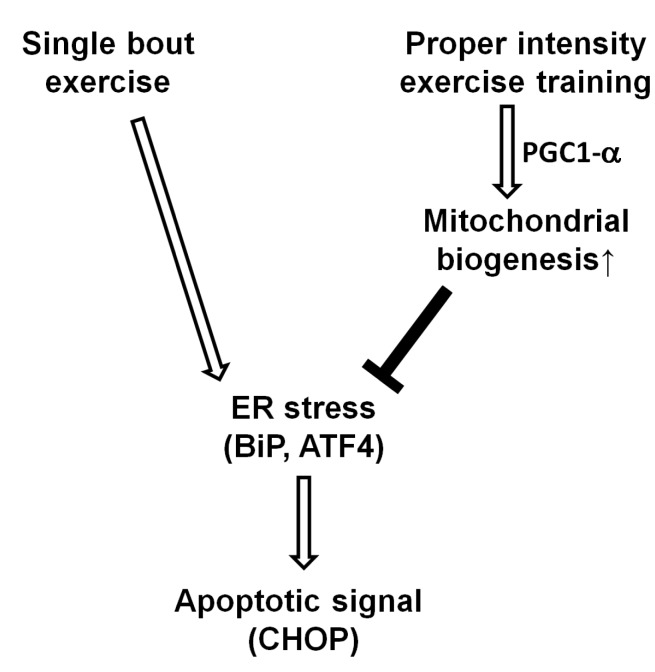
Adaptation to exercise training was also observed in the levels of the protein CHOP. If the UPR to ER stress is unsuccessful, chronic ER stress can induce ER stress-related cell death [29]. Although there are several known pathways of apoptosis resulting from ER stress, CHOP is the bestknown transcriptional factor of ER-induced cell death [26]. In addition, ATF3 and ATF4 are known to activate CHOP [30], which has pro-apoptotic activity through the downregulation of BCL-2 and upregulation of BIM, PUMA, and GADD34 [4]. Intriguingly, the level of CHOP mRNA was significantly reduced in the skeletal muscle of the trained rats and the decrease in gene expression was greater in the HIT group in comparison with the LIT group. However, while the level of CHOP was somewhat decreased in the trained groups, the difference was not significant. These results imply that regular exercise with proper intensity may diminish apoptotic signaling in skeletal muscle, and it can be considered as a protective mechanism for upcoming ER stress (Fig. 4). This protective effect seems likely to be derived from a decrease in ATF4 levels; this is consistent with a recent report that showed that the knock-down of ATF4 decreased CHOP, and subsequent apoptosis [31]. However, we were unable to detect any change in another important transcription factor, spliced XBP1 mRNA, which is also involved in ER stress-induced apoptosis (data not shown).
ER stress is known to induce mitochondrial dysfunction [8,32] and mitochondrial damage, observed by Southern blot analysis and mitochondrial copy number, is known to be associated with ER stress in skeletal muscle [15]. The expression of PGC-1α was increased in the HIT group in comparison with the control group; this is concordant with a recent report that PGC-1α is one of the key factors in the regulation of the UPR observed in mice [16]. Oxidative stress is a well-known factor in inducing ER stress [5] and is considered a local messenger between the ER and mitochondria [32]. However, exercise is known to induce ROS scavengers such as superoxide dismutase and catalase [14]. Although we did not observe the ROS and their scavengers, adapted UPR and increased mitochondrial gene expression may be related to oxidative stress in our study. In addition, ROS have been reported to be involved in the regulation of PGC-1α in skeletal muscle [33] and antioxidant supplementation has been observed inhibiting the exercise-induced increase in PGC-1α [34]. Therefore, we can assume that the ROS generated during exercise training stimulated mitochondrial biogenesis and contributed to the adaptation to, and preconditioning against, further damage. UCP1 and UCP3 are known to respond rapidly to biological stimuli such as energy depletion, and the level of UCP3 in a trained human did not show any increase after acute exercise [35]. Therefore, relatively lower gene expression of UCP3 in the HIT group in comparison with the LIT group seems understandable as a more adapted status is reached in skeletal muscle as a result of more intense training. Misfolded protein can be degraded by the ubiquitin-proteasome pathway and autophagy [5]; as a result, further investigation of these two pathways will provide more information to gain clearer insight into exercise training and the adaptation mechanisms in skeletal muscle. Although the role of PGC-1α in mitochondrial biogenesis is well known, it is also involved in fiber-type switching, enhanced fatty acid oxidation, and angiogenesis [21]. Therefore, the relationship of these effects with ER stress should also be the focus of further study.
In summary, we found that 5-week high-intensity exercise training resulted in increased mitochondrial biogenesis, and decreased ER stress and apoptotic signal in the skeletal muscle tissue of rats (Fig. 4).
ACKNOWLEDGEMENTS
This research was supported by a National Research Foundation of Korea (NRF) grant to the Medical Research Center at Yeungnam University funded by the Korea government (MEST) (2005-0049417).
References
1. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529. PMID: 17565364.

2. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89–102. PMID: 22251901.

3. Lee GH, Oh HW, Lim HD, Lee W, Chae HJ, Kim HR. 4-phenylbutyric acid regulates collagen synthesis and secretion induced by high concentrations of glucose in human gingival fibroblasts. Korean J Physiol Pharmacol. 2011; 15:345–351. PMID: 22359472.

4. Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011; 91:1219–1243. PMID: 22013210.

5. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010; 140:900–917. PMID: 20303879.

6. Peter A, Weigert C, Staiger H, Machicao F, Schick F, Machann J, Stefan N, Thamer C, Häring HU, Schleicher E. Individual stearoyl-coa desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009; 58:1757–1765. PMID: 19478146.

7. Cash JG, Kuhel DG, Basford JE, Jaeschke A, Chatterjee TK, Weintraub NL, Hui DY. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem. 2012; 287:27876–27884. PMID: 22730380.

8. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306:457–461. PMID: 15486293.

9. Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007; 293:E1656–E1662. PMID: 17911343.
10. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006; 368:1673–1679. PMID: 17098085.

11. Sherman WM, Friedman JE, Gao JP, Reed MJ, Elton CW, Dohm GL. Glycemia and exercise training alter glucose transport and GLUT4 in the Zucker rat. Med Sci Sports Exerc. 1993; 25:341–348. PMID: 8455449.

12. Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993; 265:H2094–H2098. PMID: 8285249.

13. Jong-Yeon K, Hickner RC, Dohm GL, Houmard JA. Long- and medium-chain fatty acid oxidation is increased in exercisetrained human skeletal muscle. Metabolism. 2002; 51:460–464. PMID: 11912554.

14. McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001; 280:C621–C627. PMID: 11171582.

15. Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013; 8:e54059. PMID: 23342074.

16. Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011; 13:160–169. PMID: 21284983.

17. Kim HJ, Jamart C, Deldicque L, An GL, Lee YH, Kim CK, Raymackers JM, Francaux M. Endoplasmic reticulum stress markers and ubiquitin-proteasome pathway activity in response to a 200-km run. Med Sci Sports Exerc. 2011; 43:18–25. PMID: 20473228.

18. Rayavarapu S, Coley W, Nagaraju K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr Rheumatol Rep. 2012; 14:238–243. PMID: 22410828.

19. Hansen D, Dendale P, van Loon LJ, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010; 40:921–940. PMID: 20942509.

20. Garekani ET, Mohebbi H, Kraemer RR, Fathi R. Exercise training intensity/volume affects plasma and tissue adiponectin concentrations in the male rat. Peptides. 2011; 32:1008–1012. PMID: 21291933.

21. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014; 281:739–749. PMID: 24237962.

22. Deldicque L, Cani PD, Delzenne NM, Baar K, Francaux M. Endurance training in mice increases the unfolded protein response induced by a high-fat diet. J Physiol Biochem. 2013; 69:215–225. PMID: 23011781.

23. Ogata T, Machida S, Oishi Y, Higuchi M, Muraoka I. Differential cell death regulation between adult-unloaded and aged rat soleus muscle. Mech Ageing Dev. 2009; 130:328–336. PMID: 19428451.

24. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005; 74:739–789. PMID: 15952902.

25. Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009; 4:e4170. PMID: 19137072.

26. Deldicque L, Hespel P, Francaux M. Endoplasmic reticulum stress in skeletal muscle: origin and metabolic consequences. Exerc Sport Sci Rev. 2012; 40:43–49. PMID: 21918459.
27. Sou SN, Ilieva KM, Polizzi KM. Binding of human BiP to the ER stress transducers IRE1 and PERK requires ATP. Biochem Biophys Res Commun. 2012; 420:473–478. PMID: 22446326.

28. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003; 11:619–633. PMID: 12667446.

29. Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006; 4:e374. PMID: 17090218.

30. Xu L, Su L, Liu X. PKCδ regulates death receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis in human lung cancer cells. Mol Cancer Ther. 2012; 11:2174–2182. PMID: 22848091.

31. Rao J, Qin J, Qian X, Lu L, Wang P, Wu Z, Zhai Y, Zhang F, Li G, Wang X. Lipopolysaccharide preconditioning protects hepatocytes from ischemia/reperfusion injury (IRI) through inhibiting ATF4-CHOP pathway in mice. PLoS One. 2013; 8:e65568. PMID: 23750267.

32. Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009; 1787:1352–1362. PMID: 19527680.

33. Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009; 296:C116–C123. PMID: 19005163.
34. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009; 106:8665–8670. PMID: 19433800.

35. Noland RC, Hickner RC, Jimenez-Linan M, Vidal-Puig A, Zheng D, Dohm GL, Cortright RN. Acute endurance exercise increases skeletal muscle uncoupling protein-3 gene expression in untrained but not trained humans. Metabolism. 2003; 52:152–158. PMID: 12601624.

Fig. 1
The effects of high- and low-intensity exercise training on ER stress and the unfolded protein response pathway in the skeletal muscle tissue of rats. (A) Phosphorylation of PERK and (B) BiP were analyzed by western blotting in trained (LIT and HIT) and control rats. GAPDH was used as a control to ensure equal protein loading. Protein density was measured and represented in arbitrary units. Gene expression of ATF3 (C) and ATF4 (D) were analyzed using real-time PCR. Error bars represent the mean±SEM of six rats. *p<0.05 vs. Control, #p<0.05 vs. LIT or HIT (A. U., arbitrary unit; ATF, activating transcription factor; BiP, immunoglobulin heavy chain-binding protein; CON, control group; ER, endoplasmic reticulum; HIT, high-intensity training group; LIT, low-intensity training group; PERK, protein kinase RNA-like ER kinase; pPERK, phosphorylated PERK).
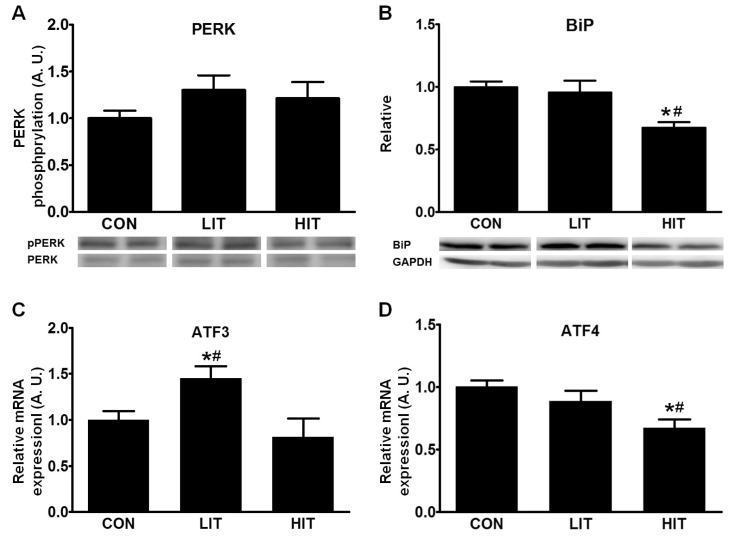
Fig. 2
The effect of high- and low-intensity exercise training on CHOP in the skeletal muscle tissue of rats. Gene expression (A) and protein level (B) were analyzed using real-time PCR and western blotting in trained (LIT and HIT) and control rats. GAPDH was used as a control to ensure equal protein loading. mRNA and protein density were measured and represented in arbitrary units. Gene expression of CHOP was normalized by the expression of GAPDH. Error bars represent the mean±SEM of six rats. *p<0.05 vs. Control, #p<0.05 vs LIT or HIT (A. U., arbitrary unit; CHOP, C/EBP-homologous protein; CON, control group; HIT, high-intensity training group; LIT, low-intensity training group).
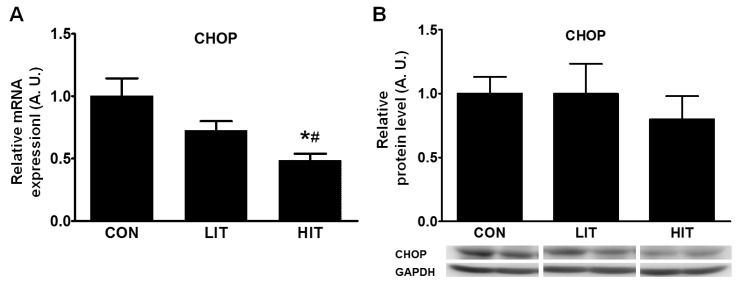
Fig. 3
Mitochondrial gene expression following high- and low-intensity exercise training in the skeletal muscle tissue of rats. Gene expression of PGC-1α (A) and UCP3 (B) were analyzed using real-time PCR in trained (LIT and HIT) and control rats. GAPDH was used as a control to ensure equal protein loading. Protein density was measured and represented in arbitrary units. Mitochondrial gene expression was normalized by the expression of GAPDH. Error bars represent the mean±SEM of six rats. *p<0.05 vs. Control (A. U., arbitrary unit; CON, control group; HIT, high-intensity training group; LIT, low-intensity training group; PGC-1α, peroxisome proliferator-activator receptor gamma coactivator-1 alpha; UCP3, mitochondrial uncoupling protein 3).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download