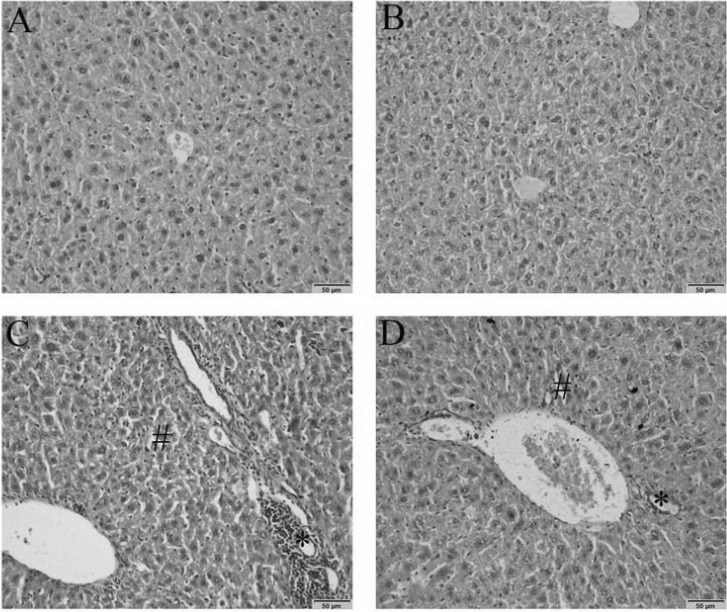
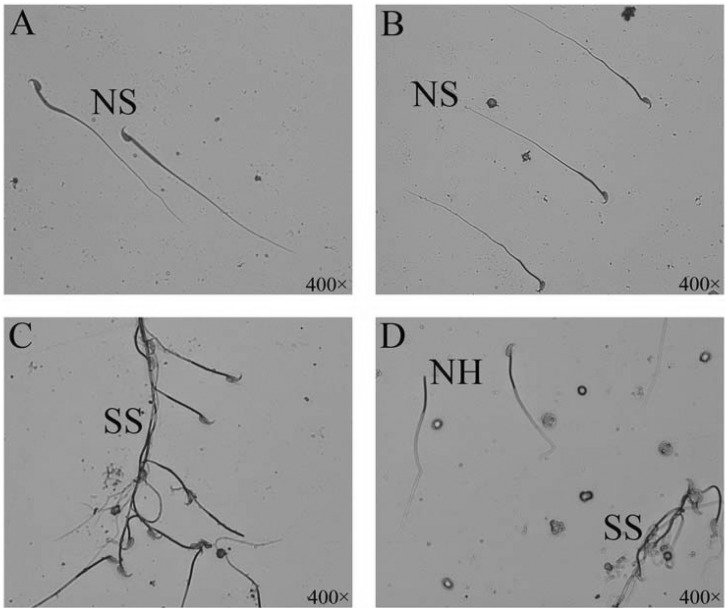
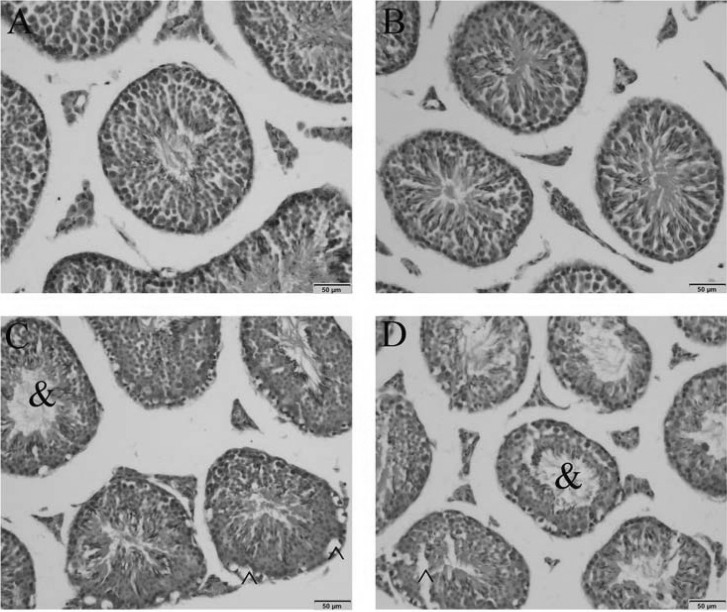
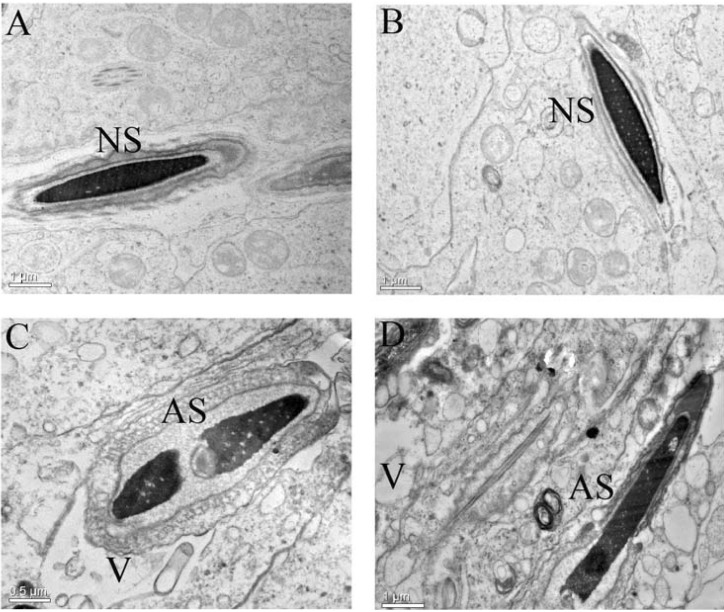
1. Boucher BJ, Mannan N. Metabolic effects of the consumption of
Areca catechu. Addict Biol. 2002; 7:103–110. PMID:
11900629.
2. Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002; 7:77–83. PMID:
11900626.

3. Mondadori C, Hengerer B, Ducret T, Borkowski J. Delayed emergence of effects of memory-enhancing drugs: implications for the dynamics of long-term memory. Proc Natl Acad Sci U S A. 1994; 91:2041–2045. PMID:
8134347.

4. Sullivan RJ, Allen JS, Otto C, Tiobech J, Nero K. Effects of chewing betel nut (
Areca catechu) on the symptoms of people with schizophrenia in Palau, Micronesia. Br J Psychiatry. 2000; 177:174–178. PMID:
11026959.
5. Stich HF, Stich W, Lam PP. Potentiation of genotoxicity by concurrent application of compounds found in betel quid: arecoline, eugenol, quercetin, chlorogenic acid and Mn
2+. Mutat Res. 1981; 90:355–363. PMID:
7335107.
6. Deng YT, Chen HM, Cheng SJ, Chiang CP, Kuo MY. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcumin. Oral Oncol. 2009; 45:e99–e105. PMID:
19457704.

7. Thangjam GS, Agarwal P, Balapure AK, Rao SG, Kondaiah P. Regulation of extracellular matrix genes by arecoline in primary gingival fibroblasts requires epithelial factors. J Periodontal Res. 2009; 44:736–743. PMID:
19438976.

8. Chou WW, Guh JY, Tsai JF, Hwang CC, Chen HC, Huang JS, Yang YL, Hung WC, Chuang LY. Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology. 2008; 243:1–10. PMID:
17997002.

9. Kumpawat K, Deb S, Ray S, Chatterjee A. Genotoxic effect of raw betel-nut extract in relation to endogenous glutathione levels and its mechanism of action in mammalian cells. Mutat Res. 2003; 538:1–12. PMID:
12834749.

10. Panigrahi GB, Rao AR. Influence of caffeine on arecoline-induced SCE in mouse bone-marrow cells
in vivo. Mutat Res. 1983; 122:347–353. PMID:
6656817.
11. Dasgupta R, Saha I, Pal S, Bhattacharyya A, Sa G, Nag TC, Das T, Maiti BR. Immunosuppression, hepatotoxicity and depression of antioxidant status by arecoline in albino mice. Toxicology. 2006; 227:94–104. PMID:
16945459.

12. Wu PF, Chiang TA, Chen MT, Lee CP, Chen PH, Ko AM, Yang KJ, Chang PY, Ke DS, Ko YC. A characterization of the antioxidant enzyme activity and reproductive toxicity in male rats following sub-chronic exposure to areca nut extracts. J Hazard Mater. 2010; 178:541–546. PMID:
20202746.

13. Lee SE, Song HJ, Park SY, Nam Y, Min CH, Lee do Y, Jeong JY, Ha HS, Kim HJ, Whang WK, Jeong JH, Kim IK, Kim HR, Min YS, Sohn UD. Effect of ECQ on Iodoacetamide-Induced Chronic Gastritis in Rats. Korean J Physiol Pharmacol. 2013; 17:469–477. PMID:
24227950.

14. Noorani AA, Kale MK. Pretreatment of albino rats with methanolic fruit extract of
Randia Dumetorum (
L.) protects against alcohol induced liver damage. Korean J Physiol Pharmacol. 2012; 16:125–130. PMID:
22563258.
15. Mongi S, Mahfoud M, Amel B, Kamel J, Abdelfattah el F. Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male Wistar rats. Ecotoxicol Environ Saf. 2011; 74:1765–1769. PMID:
21514672.

16. Wilson JX. The physiological role of dehydroascorbic acid. FEBS Lett. 2002; 527:5–9. PMID:
12220624.

17. John S, Kale M, Rathore N, Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem. 2001; 12:500–504. PMID:
11834209.

18. Cemek M, Buyukben A, Buyukokuroglu ME, Aymelek F, Tur L. Protective roles of vitamin E (a-tocopherol), selenium and vitamin E plus selenium in organophosphate toxicity in vivo: A comparative study. Pestic Biochem Physiol. 2010; 96:113–118.
19. Kalender S, Kalender Y, Ates A, Yel M, Olcay E, Candan S. Protective role of antioxidant vitamin E and catechin on idarubicin-induced cardiotoxicity in rats. Braz J Med Biol Res. 2002; 35:1379–1387. PMID:
12426639.

20. Kalender S, Kalender Y, Ogutcu A, Uzunhisarcikli M, Durak D, Açikgoz F. Endosulfan-induced cardiotoxicity and free radical metabolism in rats: the protective effect of vitamin E. Toxicology. 2004; 202:227–235. PMID:
15337585.

21. Kalender S, Ogutcu A, Uzunhisarcikli M, Açikgoz F, Durak D, Ulusoy Y, Kalender Y. Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology. 2005; 211:197–206. PMID:
15925023.

22. Serbecic N, Beutelspacher SC. Anti-oxidative vitamins prevent lipid-peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005; 320:465–475. PMID:
15838641.

23. Stoyanovsky DA, Goldman R, Darrow RM, Organisciak DT, Kagan VE. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr Eye Res. 1995; 14:181–189. PMID:
7796601.

24. Uzunhisarcikli M, Kalender Y. Protective effects of vitamins C and E against hepatotoxicity induced by methyl parathion in rats. Ecotoxicol Environ Saf. 2011; 74:2112–2118. PMID:
21782244.

25. Dahdouh F, Kechrid Z, Djebar MR. Beneficial Effects of Vitamins (C+E) Supplementation against Nickel-induced Hepatotoxicity in Mice. Adv Biores. 2013; 4:67–76.
26. Acharya UR, Mishra M, Patro J, Panda MK. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol. 2008; 25:84–88. PMID:
18065194.

27. Pradhan SN, Maickel RS. Pharmacology in Medicine Principles and Practice. USA: SP Press International Inc.;1986. p. 144.
28. Saha I, Chatterjee A, Mondal A, Maiti BR, Chatterji U. Arecoline augments cellular proliferation in the prostate gland of male Wistar rats. Toxicol Appl Pharmacol. 2011; 255:160–168. PMID:
21741983.

29. Zhang H, Wang H, Ji YL, Ning H, Yu T, Zhang C, Zhang Y, Zhao XF, Wang Q, Liu P, Meng XH, Xu DX. Lactational fenvalerate exposure permanently impairs testicular development and spermatogenesis in mice. Toxicol Lett. 2009; 191:47–56. PMID:
19683566.

30. World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press;1999. p. 128.
31. Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol In Vitro. 1998; 12:699–704. PMID:
20654459.

32. Chang MC, Ho YS, Lee PH, Chan CP, Lee JJ, Hahn LJ, Wang YJ, Jeng JH. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis. 2001; 22:1527–1535. PMID:
11532876.

33. Ebuehi OA, Ogedegbe RA, Ebuehi OM. Oral administration of vitamin C and vitamin E ameliorates lead-induced hepatotoxicity and oxidative stress in the rat brain. Nig Q J Hosp Med. 2012; 22:85–90. PMID:
23175903.
34. Prabu SM, Shagirtha K, Renugadevi J. Naringenin in combination with vitamins C and E potentially protects oxidative stress-mediated hepatic injury in cadmium-intoxicated rats. J Nutr Sci Vitaminol (Tokyo). 2011; 57:177–185. PMID:
21697638.

35. Adikwu E, Deo O. Hepatoprotective effect of vitamin C (ascorbic acid). Pharmacol Pharm. 2013; 4:84–92.

36. Joshi SC, Mathur R, Gulati N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol Ind Health. 2007; 23:439–444. PMID:
18536496.

37. ElMazoudy RH, Attia AA, El-Shenawy NS. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic Biochem Physiol. 2011; 101:175–181.

38. El-Missiry MA. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999; 124:233–237. PMID:
10661714.

39. Marchlewicz M, Wiszniewska B, Gonet B, Baranowska-Bosiacka I, Safranow K, Kolasa A, Głabowski W, Kurzawa R, Jakubowska K, Rać ME. Increased lipid peroxidation and ascorbic acid utilization in testis and epididymis of rats chronically exposed to lead. Biometals. 2007; 20:13–19. PMID:
16699871.

40. Ghosh D, Das UB, Misro M. Protective role of alpha-tocopherol-succinate (provitamin-E) in cyclophosphamide induced testicular gametogenic and steroidogenic disorders: a correlative approach to oxidative stress. Free Radic Res. 2002; 36:1209–1218. PMID:
12592673.
41. Uzun FG, Kalender S, Durak D, Demir F, Kalender Y. Malathion-induced testicular toxicity in male rats and the protective effect of vitamins C and E. Food Chem Toxicol. 2009; 47:1903–1908. PMID:
19442699.