Abstract
We have examined the effects of certain mutations of the selectivity filter and of the membrane helix M2 on Ba2+ blockage of the inward rectifier potassium channel, Kir 2.1. We expressed mutant and wild type murine Kir 2.1 in Chinese hamster ovary (CHO) cells and used the whole cell patch-clamp technique to record K+ currents in the absence and presence of externally applied Ba2+. Wild type Kir2.1 was blocked by externally applied Ba2+ in a voltage and concentration dependent manner. Mutants of Y145 in the selectivity filter showed little change in the kinetics of Ba2+ blockage. The estimated Kd(0) was 108 μM for Kir2.1 wild type, 124 μM for a concatameric WT-Y145V dimer, 109 μM for a WT-Y145L dimer, and 267 μM for Y145F. Mutant channels T141A and S165L exhibit a reduced affinity together with a large reduction in the rate of blockage. In S165L, blockage proceeds with a double exponential time course, suggestive of more than one blocking site. The double mutation T141A/S165L dramatically reduced affinity for Ba2+, also showing two components with very different time courses. Mutants D172K and D172R (lining the central, aqueous cavity of the channel) showed both a decreased affinity to Ba2+ and a decrease in the on transition rate constant (kon). These results imply that residues stabilising the cytoplasmic end of the selectivity filter (T141, S165) and in the central cavity (D172) are major determinants of high affinity Ba2+ blockage in Kir 2.1.
REFERENCES
Abrams CJ., Davies NW., Shelton PA., Stanfield PR. The role of a single aspartate residue in ionic selectivity and block of a murine inward rectifier K+ channel Kir2.1. J Physiol. 493:643–649. 1996.
Alagem N., Dvir M., Reuveny E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 534:381–393. 2001.
Dart C., Leyland ML., Barrett-Jolley R., Shelton PJ., Spencer PJ., Conley EC., Sutcliffe MJ., Stanfield PR. The dependence of Ag+ block of a potassium channel, murine Kir2.1, on a cysteine residue in the selectivity filter. J Physiol. 511:15–24. 1998.
Doring F., Derst C., Wischmeyer E., Karschin C., Schneggenburger R., Daut J., Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci. 18:8625–8636. 1998.
Doyle DA., Morais CJ., Pfuetzner RA., Kuo A., Gulbis JM., Cohen SL., Chait BT., Mackinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. 1998.
Duprat F., Lesage F., Fink M., Reyes R., Heurateaux C., Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 17:5464–5471. 1997.
Fakler B., Brandle U., Bond CH., Glowatzki E., Konig C., Adelman JP., Zenner HP., Ruppersberg JP. A structural determinant of differential sensitivity of cloned inward rectifier K+ channels to intracellular spermine. FEBS Lett. 356:199–203. 1994.
Fakler B., Brandle U., Glowatzki E., Weidemann S., Zenner HP., Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 80:149–154. 1995.
Ficker E., Taglialatela M., WIBLE BA., Henley CM., Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 266:1068–1072. 1994.
Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 279:167–185. 1978.

Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 292:251–265. 1979.

Harris RE., Larsson HP., Isakoff EY. A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys J. 74:1808–1820. 1998.
Heginbotham L., Lu Z., Abramson T., Mackinnon R. Mutations in the K+ channel signature sequence. Biophys J. 66:1061–1067. 1994.
Hidalgo P., Mackinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 268:307–310. 1995.
Hurst RS., Toro L., Stefani E. Molecular determinants of external barium block in Shaker potassium channels. FEBS Lett. 388:59–65. 1996.
Jiang Y., Mackinnon R. The barium site in a potassium channel by x-ray crystallography. J Gen Physiol. 115:269–272. 2000.

Krapivinsky G., Medina I., Eng L., Krapivinsky L., Yang Y., Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron. 20:995–1005. 1998.
Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 82:488–492. 1985.

Kuo A., Gulbis JM., Antcliff JF., Rahman T., Lowe ED., Zimmer J., Cuthbertson J., Ashcroft FM., Ezaki T., Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. 2003.

Lancaster MK., Dibb KM., Quinn CC., Leach R., Lee JK., Findlay JB., Boyett MR. Residues and mechanisms for slow activation and Ba2+ block of the cardiac muscarinic K+ channel, Kir3.1/Kir3.4. J Biol Chem. 275:35831–35839. 2000.
Lee YM., Yang DK., Ashmole I., Stanfield PR., So I., Kim KW. Residues lining the pore region of the murine inward rectifier K+ channel (KIR2.1) control Ba2+ blockage. Biophysical J. 80:631A. 2001.
Leech CA., Stanfield PR. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 319:295–309. 1981.

Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romely G., Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 15:1004–1011. 1996.
Lockless SW., Zhou M., Mackinnon R. Structural and thermo-dynamic properties of selective ion binding in a K+ channel. PLoS Biology. 5:1079–1088. 2007.
Lopatin AN., Makhina EN., Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 372:366–369. 1994.

Lopatin AN., Makhina EN., Nichols CG. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J Gen Physiol. 106:923–955. 1995.

Lopatin AN., Nichols CG. [K+] Dependence of polyamine-induced rectification in inward rectifier potassium channels (IRK1, Kir2.1). J Gen Physiol. 108:105–113. 1996.
Lu T., Ting AY., Mainland J., Jan LY., Schultz PG., Yang J. Probing ion permeation and gating in a K+ channel with backbone mutations in the selectivity filter. Nature Neurosci. 4:239–246. 2001.
Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 325:156–159. 1987.
Minor DL., Masseling SJ., Jan YN., Jan LY. Transmembrane structure of an inwardly rectifying potassium channel. Cell. 96:879–891. 1999.

Navaratnam DS., Escobar L., Covarrubias M., Oberholtzer JC. Permeation properties and differential expression across the auditory receptor epithelium of an inward rectifier K+ channel cloned from the chick inner ear. J Biol Chem. 270:19238–19245. 1995.
Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 92:549–567. 1988a.

Neyton J., Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high conductance Ca2+ activated K+ channel. J Gen Physiol. 92:569–586. 1988b.
Nishida M., Cadene M., Chait BT., Mackinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 26:4005–4015. 2007.

Reuveny E., Jan YN., Jan LY. Contributions of a negatively charged residue in the hydrophobic domain of the IRK1 inwardly rectifying K+ channel to K+-selective permeation. Biophys J. 70:754–761. 1996.
Sabirov RZ., Tominaga T., Miwa A., Okada Y., Oiki S. A conserved arginine residue in the pore region of an inward rectifier K channel (IRK1) as an external barrier for cationic blockers. J Gen Physiol. 110:665–677. 1997.

Shieh RC., Chang JC., Arreola J. Interaction of Ba2+ with the pores of the cloned inward rectifier K+ channels Kir2.1 expressed in Xenopus oocytes. Biophys J. 75:2313–2322. 1998.
Shioya T., Matsuda H., Noma A. Fast and slow blockades of the inward-rectifier K+ channel by external divalent cations in guinea-pig cardiac myocytes. Pflügers Arch. 422:427–435. 1993.
So I., Ashmole I., Davies NW., Sutcliffe MJ., Stanfield PR. The K+ channel signature sequence of murine Kir2.1: mutations that affect microscopic gating but not ionic selectivity. J Physiol. 531:37–50. 2001.
So I., Ashmole I., Soh H., Park CS., Spencer PJ., Leyland M., Stanfield PR. Intrinsic gating in inward rectifier potassium channels (Kir2.1) with low polyamine affinity generated by site directed mutagenesis. Kor J Physiol Pharmacol. 7:131–142. 2003a.
So I., Ashmole I., Stanfield PR. The substates with mutants that negatively charged aspartate in position 172 was replaced with positive charge in murine inward rectifier potassium channel (murine Kir2.1). Kor J Physiol Pharmacol. 7:267–273. 2003b.
Standen NB., Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 280:169–191. 1978.

Stanfield PR., Davies NW., Shelton PA., Sutcliffe MJ., Khan IA., Brammar WJ., Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 478:1–6. 1994.
Stanfield PR., Nakajima S., Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 145:47–179. 2002.
Thompson GA., Passmore GM., Spencer PJ., Davies NW., Stanfield PR. Blockage of inward rectifier potassium channels (murine Kir2.1) by Ba2+ is influenced by an aspartate residue (D172). J Physiol. 511P:146P. 1998.
Thompson GA., Leyland ML., Ashmole I., Sutcliffe MJ., Stanfield PR. Residues beyond the selectivity filter of the K+ channel Kir2.1 regulate permeation and block by external Rb+ and Cs+. J Physiol. 526:231–240. 2000a.
Thompson GA., Leyland ML., Ashmole I., Stanfield PR. Residues in H5 and M2 of murine Kir2.1 regulate Ba2+ block. J Physiol. 526P:10S. 2000b.
Topert C., Doring F., Wischmeyer E., Karschin C., Brockhaus J., Ballanyi K., Derst C., Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci. 18:4096–4105. 1998.
Yang J., Jan YN., Jan LY. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 15:1441–1447. 1995.

Zhou H., Chepilko S., Schutt W., Choe H., Palmer LG., Sackin H. Mutations in the pore region of ROMK enhance Ba2+ block. Am J Physiol. 271:C1949–C1956. 1996.
Fig. 1.
Ba2+ blockage of wild type Kir2.1 channels. (A) Membrane currents recorded from a single CHO cell expressing the gene for Kir2.1 in response to voltage steps from a holding potential of −17 mV to test potentials ranging from +63 mV to −117 mV, using 10 mV increments. Extracellular [K+] was 70 mM, and intracellular [K+] was 140 mM. (B~D) K+ currents activated by voltage steps from −17 mV to a range of potentials between +63 and −127 mV, in the presence of 10 μM (B), 100 μM (C), 1 mM (D) [Ba2+]o. The current was reduced to 3.8±0.6% of control levels after 1,500 ms at −117 mV in the presence of 100 μM Ba2+ (n=7).
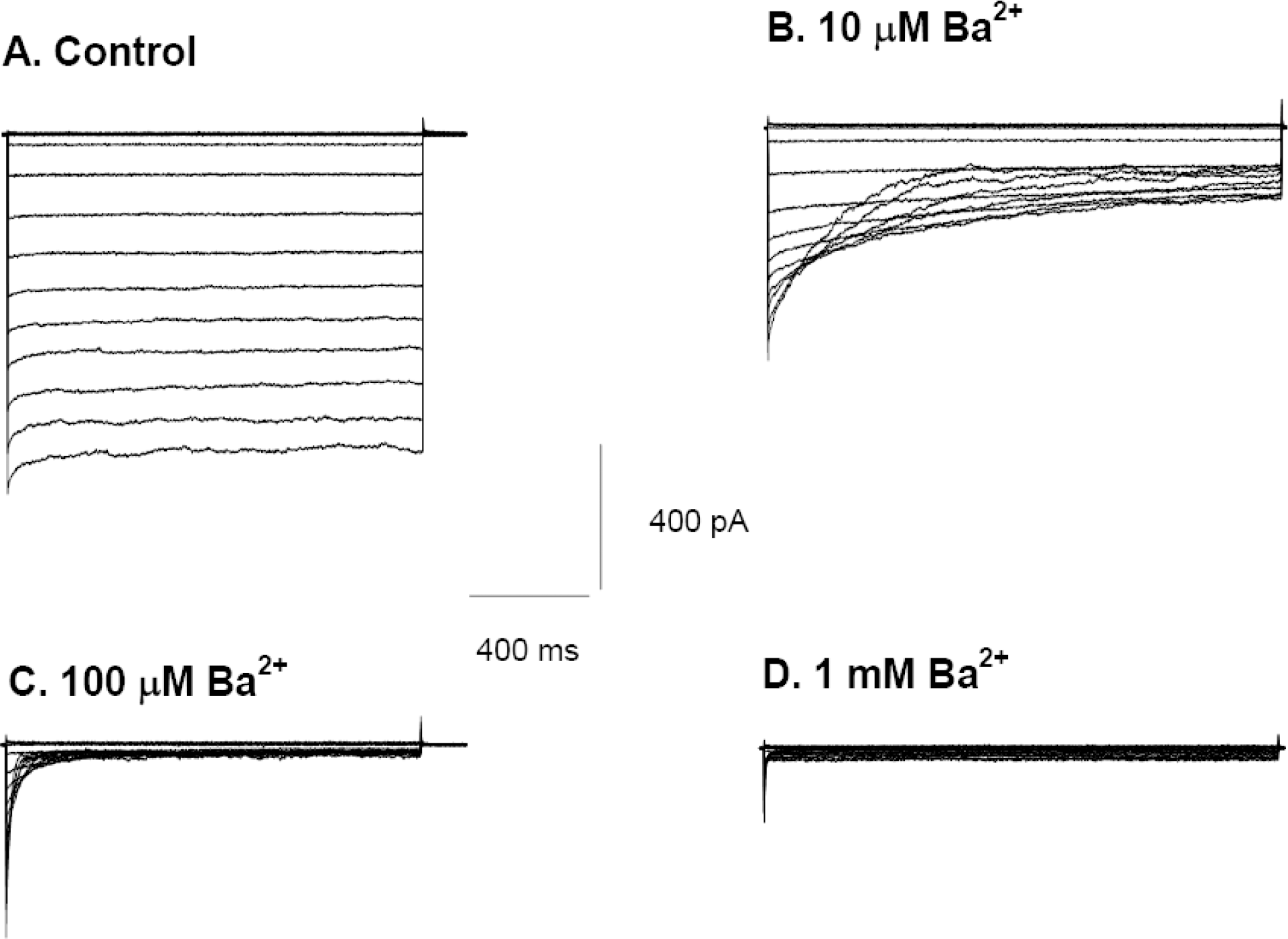
Fig. 2.
Voltage and concentration dependence of blockage by [Ba2+]o of Kir2.1. (A) Dose-response curves for extracellular Ba2+ blockage of steady-state IK at −57 mV (squares), −77 mV (triangles), −97 mV (inverted triangles), −117 mV (diamonds), −127 mV (circles). Lines are the best fits to the averaged data (n=7 for each point) using the Hill equation (Eqn. 1 of text). (B) The reciprocal of the time constant (τ) of exponential curves fitted to development of Ba2+ blockage plotted against Ba2+ concentration (n=5). Solid lines are linear fits to data using Eqn (3) of the text. Slopes (bottom to top) at −57 mV (squares), −77 mV (triangles), −97 mV (inverted triangles), −117 mV (diamonds), −127 mV (circles) were: 1.1×105 M−1s−1, 1.9×105 M−1s−1, 3.9×105 M−1s−1, 6.4×105 M−1s−1 and 9.1×105 M−1s−1, respectively.
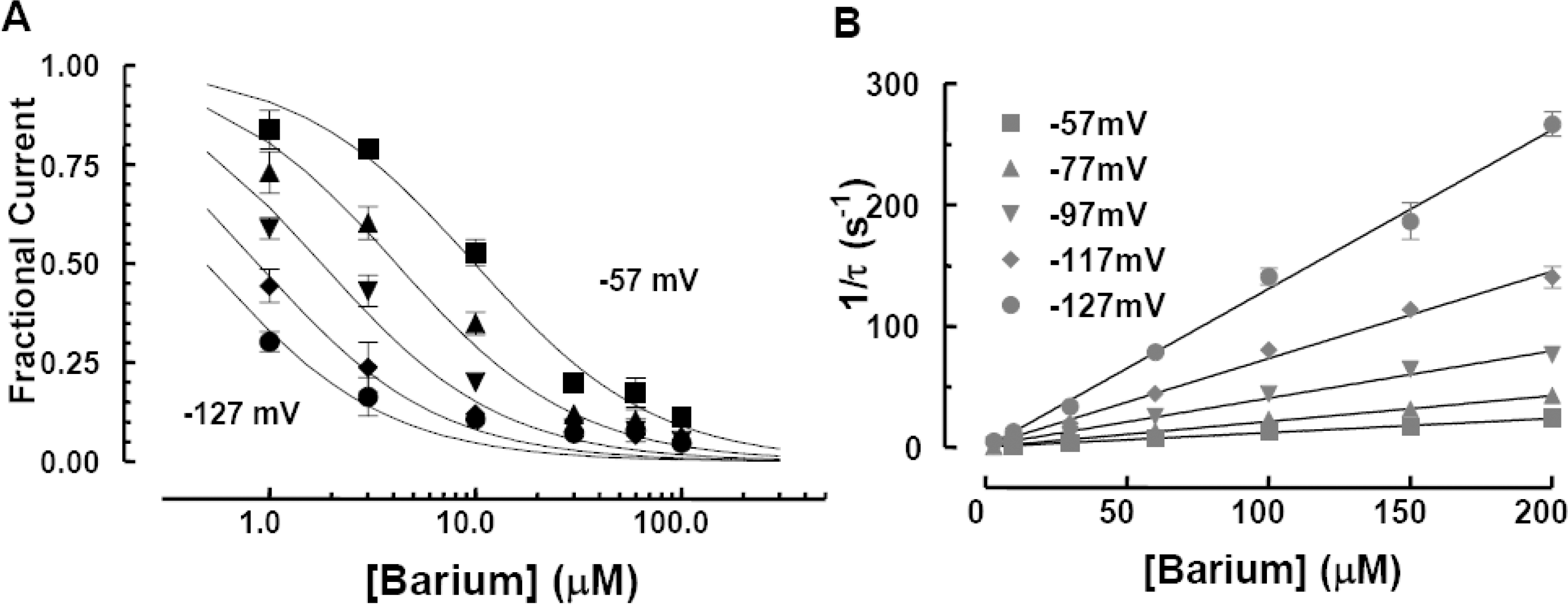
Fig. 3.
Blockage by Ba2+ is similar in Kir2.1 wild type, Y145F, WT-Y145L dimer and WT-Y145V dimer. (A) Plot of kon against membrane potential for wild type and Y145 mutants. kon values were obtained from the value of τ by fitting regression lines to Eqn (3). (B) Plot of koff against membrane potential for wild type and for Y145 mutants. koff values were obtained using Kd and kon. (C) Kd values obtained from the concentration-dependent inhibition of the steady-state currents were plotted against membrane potential. The solid line is the best fit of the data obtained at membrane potential (V) ranging from −127 mV to −47 mV, using the Woodhull equation (Eqn 4 of text).
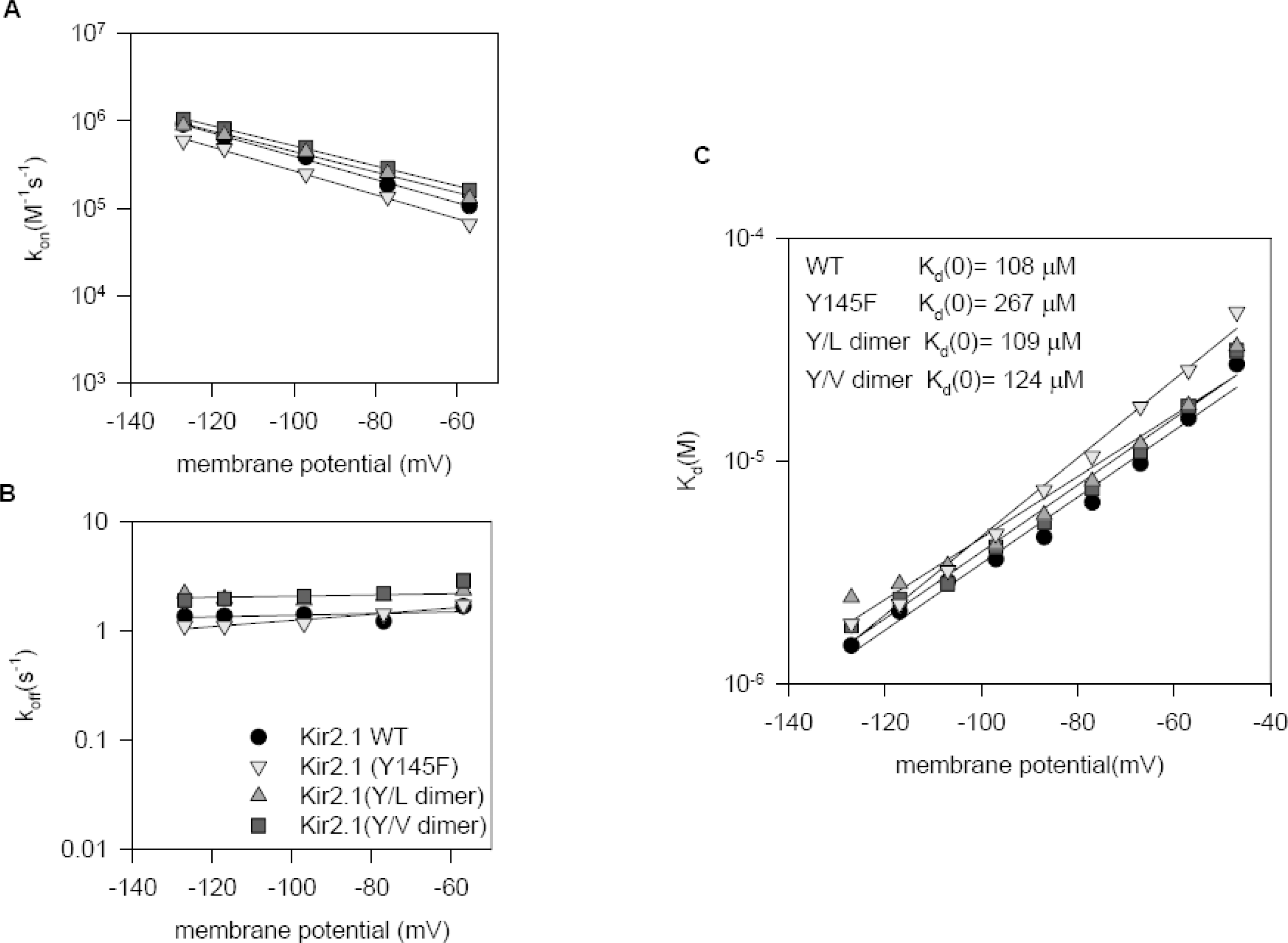
Fig. 4.
Ba2+ blockage of T141A. T141A slows block and lowers Ba2+ affinity (Kd(0)=1.02 mM; δ=0.61), primarily through a reduction in kon. (A, B) Blockage proceeds with a single exponential time course in both the wild type and mutant channel. Kir2.1 currents are shown for wild type (A) and T141A (B) in response to hyperpolarising pulses from a holding potential of −17 mV to voltages between −37 and −127 mV in 10 mV increments. (C) Relationship between the fractional steady state current (ordinate) and [Ba2+]o (abscissa) for wild type and T141A. (D) Relationship between the transition rate constants kon (above) and koff (below) plotted (ordinate) against membrane potential (abscissa) for wild type and T141A.
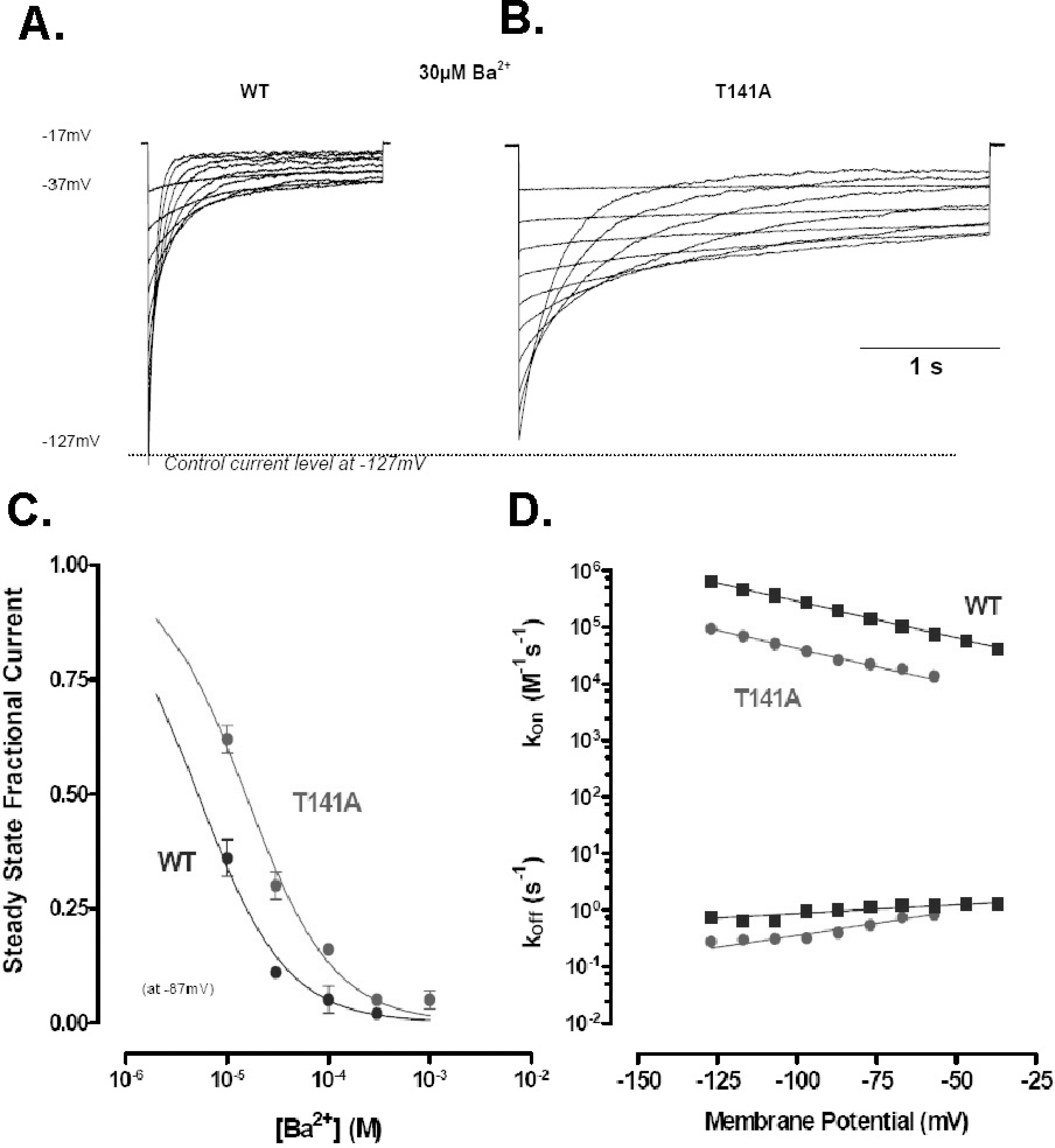
Fig. 5.
Ba2+ blockage of S165L. S165L reduces Ba2+ affinity (Kd(0)=1.04 mM; δ=0.69) and slows blockage. With 300 μM Ba2+ the time-course of block becomes biphasic. The time course of each component and the relatively high affinity for Ba2+ makes detailed analysis of kinetics difficult.
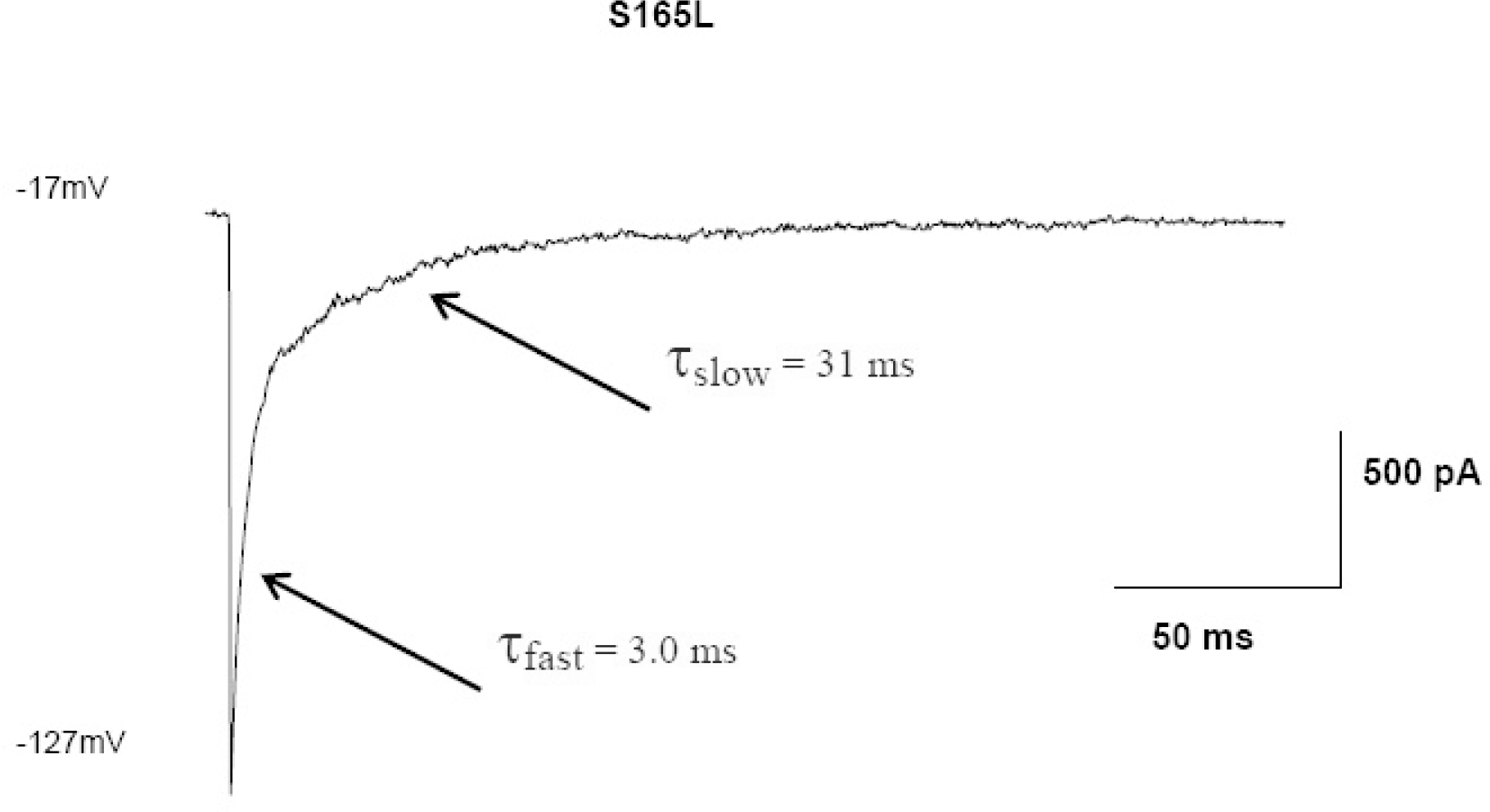
Fig. 6.
Ba2+ blockage of T141A/S165L. The double mutant T141A/S165L, which is permeable to Rb+ and Cs+, drastically reduces Ba2+ affinity, revealing two blocking sites. At high concentrations, blockage becomes dominated by the fast component allowing calculation of affinity and voltage- dependence (Kd(0)=210 mM; (δ=0.68). (A, B) Currents from T141A/S165L mutant channels obtained in the absence (A) and presence of 300 μM Ba2+ in response to hyperpolarising pulses from a holding potential of −17 mV to voltages between −37 and −127 mV. (C, D) The time constants obtained by fitting the decline in current with the sum of two exponentials and plotted (ordinates) against Ba2+ concentration for fast (C) and slow (D) components. (E) Plots of the transition rate constants (ordinate) kon (symbols in red) and koff (symbols in blue) plotted against membrane potential. kon for the faster component has the greater voltage dependence.
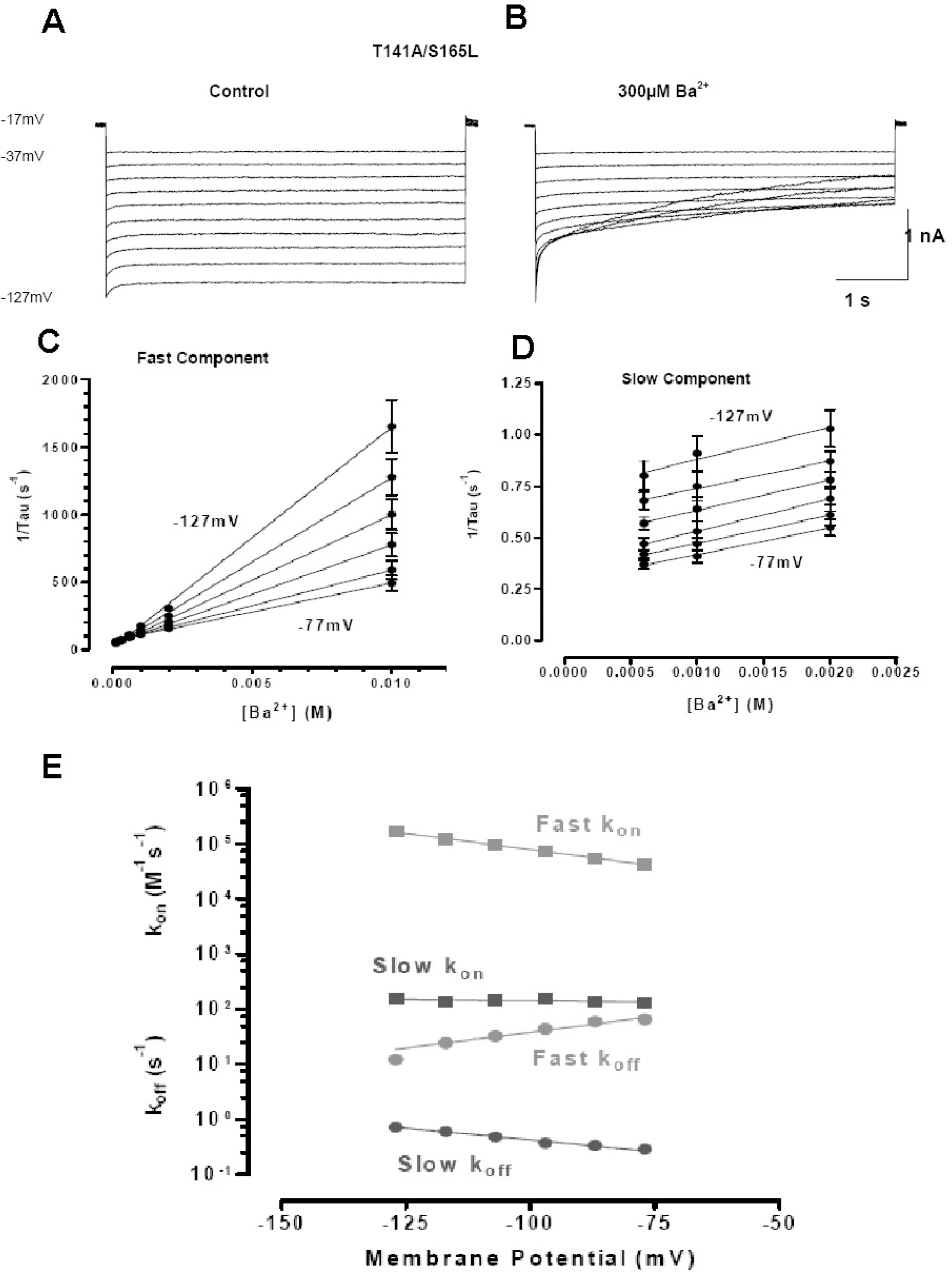
Fig. 7.
Ba2+ blockage of Kir2.1 wild type and D172R. (A, B) Membrane currents recorded from a single CHO cell expressing the gene for wild type (A) and D172R (B) in response to voltage steps from a holding potential of −17 mV to test potentials ranging from +63 to −127 mV (10 mV increments) in the presence of 30 μM Ba2+. (C, D) Currents in wild type (C) and D172R (D) with 100μM Ba2+ at the voltage indicated. The time dependence of block fitted to a single exponential.
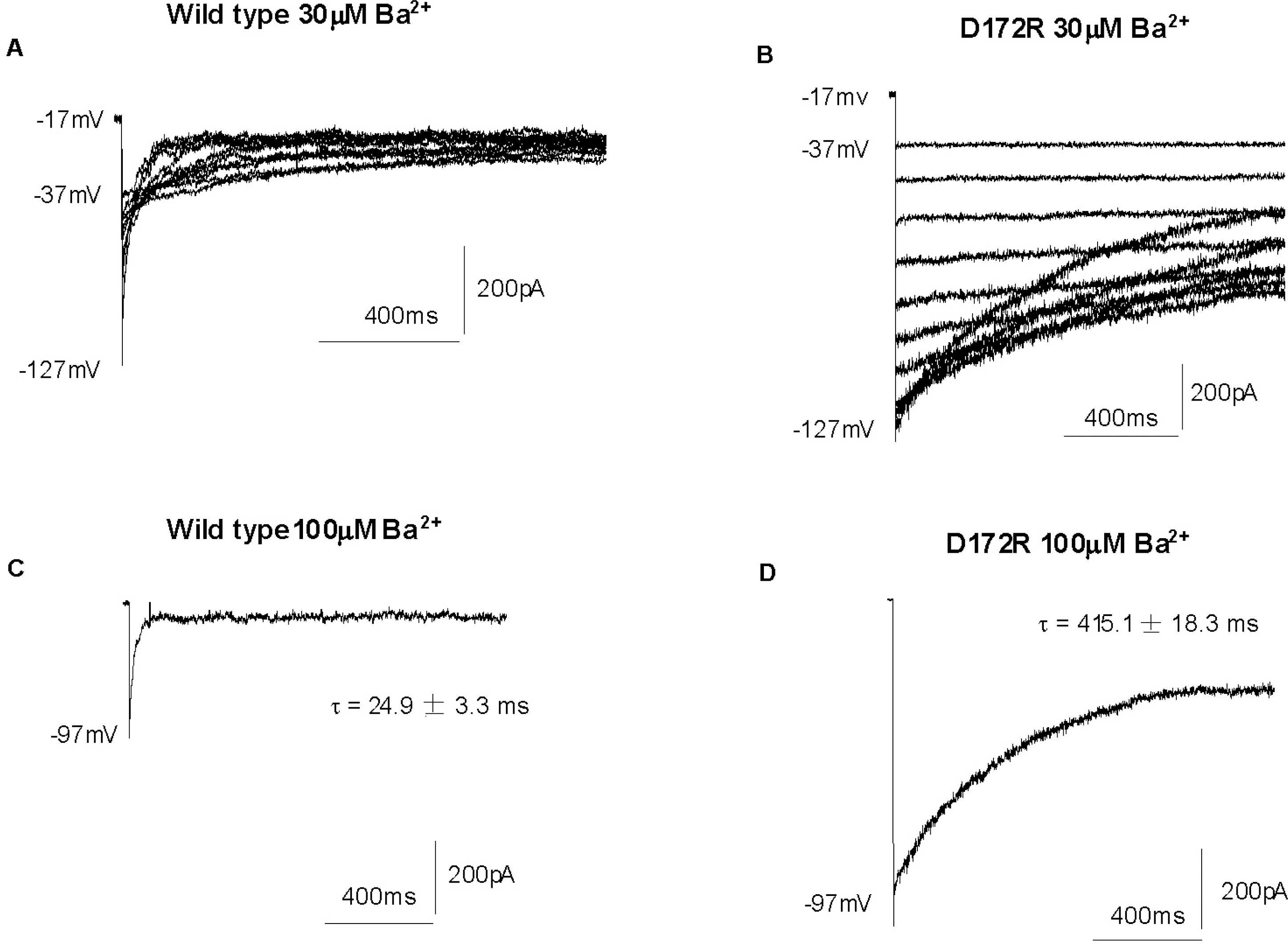
Fig. 8.
D172K and D172R mutants decreased kon and increased the Kd. (A) Plot of kon against membrane potential for wild type (circles), D172R (squares), D172K (triangles). kon values were obtained from τ by fitting regression lines to Eqn (3) of the text. (B) Plot of koff against membrane potential for wild type (circles), D172R (squares) and D172K (triangles). koff values were obtained using Kd and kon. (C) Kd values obtained from the concentration-dependent inhibition of the steady-state currents were plotted against membrane potentials. The solid line is the best fit of the data obtained at membrane potential (V) ranging from −127 mV to −47 mV, using the Woodhull equation (Eqn 4 of the text). The values obtained for Kd(0) and δ are summarized in Table 1.
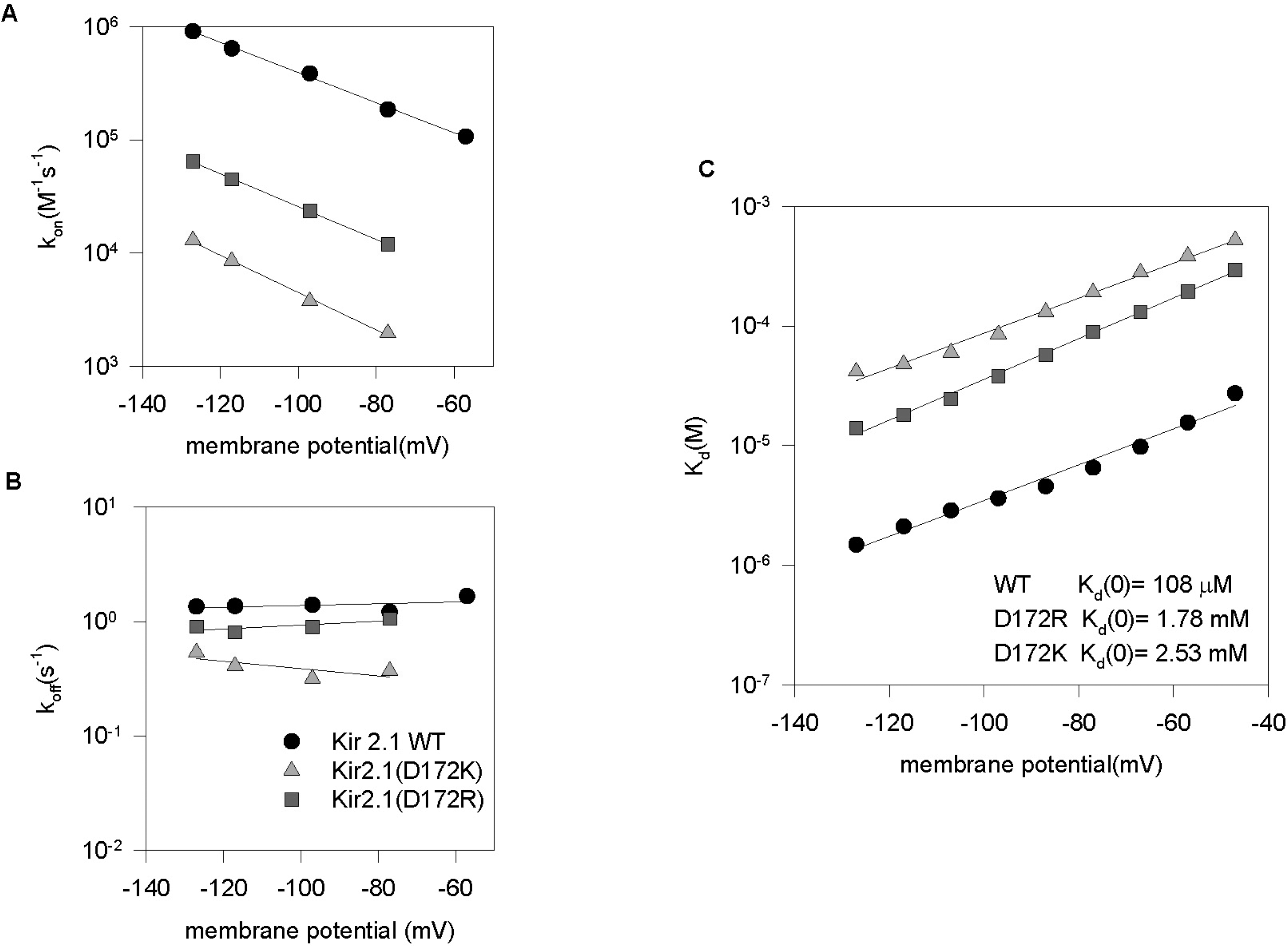
Table 1.
Ba2+ blockage in wild type and in mutant channels
Kd(0) is the dissociation constant at 0 mV and δ is the electrical distance between the outside of the membrane and the blocking site, both obtained using Eqn (4) of the text. The values for the transition rate constants kon and koff are derived using Eqn (3) of the text and are given here for a membrane potential of −127 mV.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download