Abstract
The aim of the present study was to examine the effects of ketamine, a dissociative anesthetics, on secretion of catecholamines (CA) secretion evoked by cholinergic stimulation from the perfused model of the isolated rat adrenal gland, and to establish its mechanism of action, and to compare ketamine effect with that of thiopental sodium, which is one of intravenous barbiturate anesthetics. Ketamine (30 ~ 300 μ M), perfused into an adrenal vein for 60 min, dose- and time-dependently inhibited the CA secretory responses evoked by ACh (5.32 mM), high K+ (a direct membrane-depolarizer, 56 mM), DMPP (a selective neuronal nicotinic NN receptor agonist, 100 μ M) and McN-A-343 (a selective muscarinic M1 receptor agonist, 100 μ M). Also, in the presence of ketamine (100 μM), the CA secretory responses evoked by veratridine (a voltage-dependent Na+ channel activator, 100 μM), Bay-K-8644 (an L-type dihydropyridine Ca2+ channel activator, 10 μM), and cyclopiazonic acid (a cytoplasmic Ca2+-ATPase inhibitor, 10 μM) were significantly reduced, respectively. Interestingly, thiopental sodium (100 μM) also caused the inhibitory effects on the CA secretory responses evoked by ACh, high K+, DMPP, McN-A-343, veratridine, Bay-K-8644, and cyclopiazonic acid. Collectively, these experimental results demonstrate that ketamine inhibits the CA secretion evoked by stimulation of cholinergic (both nicotinic and muscarinic) receptors and the membrane depolarization from the isolated perfused rat adrenal gland. It seems likely that the inhibitory effect of ketamine is mediated by blocking the influx of both Ca2+ and Na+ through voltage-dependent Ca2+ and Na+ channels into the rat adrenal medullary chromaffin cells as well as by inhibiting Ca2+ release from the cytoplasmic calcium store, which are relevant to the blockade of cholinergic receptors. It is also thought that, on the basis of concentrations, ketamine causes similar inhibitory effect with thiopental in the CA secretion from the perfused rat adrenal medulla.
REFERENCES
Akaike A., Mine Y., Sasa M., Takaori S. Voltage and current clamp studies of muscarinic and nicotinic excitation of the rat adrenal chromaffin cells. J Pharmacol Exp Ther. 255:333–339. 1990.
Anton AH., Sayre DF. A study of the factors affecting the aluminum oxidetrihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 138:360–375. 1962.
Azzaro AJ., Smith DJ. The inhibitory action of ketamine HCl on [3H]5-hydroxytryptamine accumulation by rat brain synaptosomal-rich fractions: comparison with [3H]catecholamine and [3H]γ-aminobutyric acid uptake. Neuropharmacology. 16:349–356. 1977.
Burgoyne RD. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 779:201–216. 1984.

Cena V., Nicolas GP., Sanchez-Garcia P., Kirpekar SM., Garcia AG. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 10:1455–1462. 1983.

Challis RA., Jones JA., Owen PJ., Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 56:1083–1086. 1991.

Cheek TR., O'Sullivan AJ., Moreton RB., Berridge MJ., Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: Distinct nicotinic and muscarinic patterns. FEBS Lett. 247:429–434. 1989.
Clanachan AS., McGrath JC. Effects of ketamine on the peripheral autonomic nervous system of the rat. Br J Pharmacol. 58:247–252. 1976.

Cohen ML., Chan SL., Way WL., Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 39:370–376. 1973.

Corssen G., Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 45:29–40. 1966.
Diaz FA., Bianco JA., Bello A., Beer N., Velarde H., Izquierdo JP., Jaen R. Effects of ketamine on canine cardiovascular function. Br J Anaesth. 48:941–946. 1976.

Domino EF., Chodoff P., Corssen G. Pharmacologic effects of CI-581, a new dissociative anesthetic in man. Clin Pharmacol Ther. 6:279–291. 1965.

Domino EF., Zsigmond EK., Domino LE., Domino KE., Kothary SP., Domino SE. Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg. 61:87–92. 1982.

Douglas WW., Kanno T., Sampson SR. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 191:107–121. 1967.
Dowdy EG., Kaya K. Studies of the mechanism of cardiovascular responses to CI-581. Anesthesiology. 29:931–943. 1968.

Gallagher JP., Dun N., Higashi H., Nisihi S. Actions of ketamine on synaptic transmission in frog sympathetic ganglia. Neuropharmacology. 15:139–143. 1976.

Garcia AG., Sala F., Reig JA., Viniegra S., Frias J., Fonteriz R., Gandia L. Dihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 309:69–71. 1984.

Garty M., Deka-Starosta A., Stull R., Kopin IJ., Goldstein DS. Effects of general anesthetics on plasma levels of catechols in intact and in adrenal-demedullated rats. Biogenic Amines. 7:435–443. 1990.
Gemperle M., Szappanyos G., Rifat K. Neuroleptanesthesia in gerontosurgery. Int Anesthesiol Clin. 11:125–138. 1973.

Goeger DE., Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 38:3995–4003. 1989.
Goldberg AH., Keane PW., Phear WP. Effects of ketamine on contractile performance and excitability of isolated heart muscle. J Pharmacol Exp Ther. 175:388–394. 1970.
Graf BM., Vicenzi MN., Martin E., Bosnjak ZJ., Stowe DF. Ketamine has stereospecific effects in the isolated perfused guinea pig heart. Anesthesiology. 82:1426–1437. 1995.

Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 31:2991–2998. 1982.

Hara K., Minami K., Ueno S., Toyohira Y., Tsutsui M., Shigematsu A., Yanagihara N. Up-regulation of noradrenaline transporter in response to prolonged exposure to ketamine. Naunyn Schmiedeberg's Arch Pharmacol. 365:406–412. 2002.

Hara K., Yanagihara N., Minami K., Ueno S., Toyohira Y., Sata T., Kawamura M., Bruss M., Bonisch H., Shigematsu A., Izumi F. Ketamine interacts with the noradrenaline transporter at a site partly overlapping the desipramine binding site. Naunyn-Schmiedeberg's Arch Pharmacol. 358:328–333. 1998.

Harish OE., Kao LS., Raffaniello R., Wakade AR., Schneider AS. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 48:1730–1735. 1987.

Hensel I., Braun U., Kettler D., Knoll D., Martel J., Paschen K. Studies on circulatory and metabolic changes during ketamine anaesthesia. Anaesthesist. 21:44–49. 1972.
Idvall J., Ahlgren I., Aronsen KF., Stenberg P. Ketamine infusions: pharmacokinetics and clinical effects. Br J Anaesth. 51:1167–1173. 1979.

Ilno M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 94:363–383. 1989.
Inoue M., Kuriyama H. Muscarinic receptor is coupled with a cation channel through a GTP-binding protein in guinea-pig chromaffin cells. J Physiol (Lond). 436:511–529. 1991.

Juang MS., Yonemura K., Morita T., Tanaka T. Ketamine acts on the peripheral sympathetic nervous system of guinea pigs. Anesth Analg. 59:45–49. 1980.

Kidokoro Y., Miyazaki S., Ozawa S. Acetylcholine-induced membrane depolarization and potential fluctuations in the rat adrenal chromaffin cell. J Physiol. 324:203–220. 1982.

Kitagawa H., Yamazaki T., Akiyama T., Yahagi N., Kawada T., Mori H., Sunagawa K. Modulatory effects of ketamine on catecholamine efflux from in vivo cardiac sympathetic nerve endings in cats. Neurosci Lett. 324:232–236. 2002.

Klose R., Peter K. Clinical studies on single-drug anesthesia using ketamine in patients with burns. Anaesthesist. 22:121–126. 1973.
Knight DE., Baker PF. Observations on the muscarinic activation of catecholamine secretion in the chicken adrenal. Neuroscience. 19:357–366. 1986.

Kolka MA., Elizondo RS., Weinberg RP. Sympathoadrenal responses to cold and ketamine anesthesia in the rhesus monkey. J Appl Physiol. 54:896–900. 1983.

Kreuscher H., Gauch H. The effect of phencylidine derivatives ketamine (CI 581) on the cardiovascular system of the man. Anaesthesist. 16:229–233. 1967.
Ladona MG., Aunis D., Gandia AG., Garcia AG. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 48:483–490. 1987.

Lim DY., Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 27:53–67. 1991.
Lim DY., Kim CD., Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 15:115–125. 1992.

Mahmoodi V., Byrne AJ., HeaIy TE., Hussain SZ. Effect of ketamine on transmission in sympathetic ganglia. Br J Anaesth. 52:371–375. 1980.

Maleque MA., Warnick JE., Albuquerque EX. The mechanism and site of action of ketamine on skeletal muscle. J Pharmacol Exp Ther. 219:638–645. 1981.
McCarthy DA., Chen G., aup DH., Ensor C. General anesthetic and other pharmacological properties of 2-(O-chlorophenyl)-2-methylamino cyclohexane HCl (CI-581). J New Drugs. 5:21–33. 1965.
McGrath JC., MacKenzie JE., Millar RA. Circulatory responses to ketamine: dependence on respiratory pattern and background anaesthesia in the rabbit. Br J Anaesth. 47:1149–1156. 1975.

Misbahuddin M., Isosaki M., Houchi H., Oka M. Muscarinic receptor-mediated increase in cytoplasmic free Ca2+ in isolated bovine adrenal medullary cells. Effects of TMB-8 and phorbol ester TPA. FEBS Lett. 190:25–28. 1985.
Nakazato Y., Ohga A., Oleshansky M., Tomita U., Yamada Y. Voltage-independent catecholamine release mediated by the activation of muscarinic receptors in guinea-pig adrenal glands. Br J Pharmacol. 93:101–109. 1988.

Oka M., Isosaki M., Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. Usdin E, Kopin IJ, Brachas J, editors. ed,. Catecholamines: Basic and Clinical frontiers. Pergamon Press;Oxford: p. p. 70–72. 1979.

Okamoto T., Okutani R., Tashiro C. The dual effect of ketamine on dopamine release from rat pheochromocytoma (PC-12) cells. Masui. 45:1083–1087. 1996.
Purifoy JA., Holz RW. The effects of ketamine, phencyclidine and lidocaine on catecholamine secretion from cultured bovine adrenal chromaffin cells. Life Sci. 35:1851–1857. 1984.
Rust M., Landauer B., Kolb E. Ketamine–a suitable agent for emergency situations. Anaesthesist. 27:205–212. 1978.
Schwartz DA., Horwitz LD. Effects of ketamine on left ventricular performance. J Pharmacol Exp Ther. 194:410–414. 1975.

Seidler NW., Jona I., Vegh N., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasimc reticulum. J Biol Chem. 264:17816–17823. 1989.
Sumikawa K., Matsumoto T., Amenomori Y., Hkano H., Amakata Y. Selective actions of intravenous anesthetics on nicotinic- and muscarinic-receptor-mediated response of the dog adrenal medulla. Anesthesiology. 59:412–416. 1983.
Suzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 107:134–140. 1992.
Takara H., Wada A., Arita M., Sumikawa K., Izumi F. Ketamine inhibits 45Ca influx and catecholamine secretion by inhibiting 22Na influx in cultured bovine adrenal medullary cells. Eur J Pharmacol. 125:217–224. 1986.
Tallarida RJ., Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed.Speringer-Verlag;New York: p. p. 132. 1987.
Taube HD., Montel H., Hau G., Starke K. Phencyclidine and ketamine: comparison with the effect of cocaine on the noradrenergic neurones of the rat brain cortex. Naunyn-Schmiedeberg's Arch Pharmacol. 291:47–54. 1975.

Traber DL., Wilson RD., Priano LL. Differentiation of the cardiovascular effects of CI-581. Anesth Analg. 47:769–778. 1968.

Uceda G., Artalejo AR., Lopez MG., Abad F., Neher E., Garcia AG. Ca2+-activated K+ channels modulate muscarinic secretion in cat chromaffin cells. J Physiol. 454:213–230. 1992.
Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 106:208–214. 1992.
Virtue RW., Alanis JM., Mori M., Lafargue RT., Vogal JHK., Metcalf DR. An anesthetic agent: 2-(O-chlorophenyl)-2-methylamino cyclohexane HCl (CI-581). Anesthesiology. 28:823–833. 1967.
Volle RL., Alkadhi KA., Branisteanu DD., Reynolds LS., Epstein PM., Amilowitz H., Lambert JJ., Henderson EG. Ketamine and ditran block end-plate ion conductance and [3H]phencyclidine binding to electric organ membrane. J Pharmacol Exp Ther. 221:570–576. 1982.
Wada A., Izumi F., Yanagihara N., Kobayashi H. Modulation by oubain and diphenylhydantoin of veratridine-induced 22Na influx and its relation to 45Ca influx and secretion of catecholamines in cultured bovine adrenal medullary cells. Naunyn-Schmiedberg's Arch Pharmacol. 328:273–278. 1985a.
Wada A., Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 15:283–292. 1985b.
Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 313:463–480. 1981.

Wieber J., Gugler R., Ohuchi T., Oka M. Pharmacokinetics of ketamine in man. Anesthesist. 24:260–283. 1975.
Fig. 1.
Concentration-dependent effects of ketamine on the secretory responses of catecholamines (CA) from the perfused rat adrenal glands evoked by acetylcholine (ACh, Upper) and by high K+ (Lower). CA secretion by a single injection of ACh (5.32×10−3 M) or K+ (56 mM) in a volume of 0.05 ml was evoked at 15 min intervals after preloading with 30, 100 and 300 μM of ketamine, respectively, for 60 min as indicated at an arrow mark. Numbers in the parenthesis indicate number of rat adrenal glands. Vertical bars on the columns represent the standard error of the mean (S.E.M.). Ordinate: the amounts of CA secreted from the adrenal gland (% of control). Abscissa: collection time of perfusate (min). Statistical difference was obtained by comparing the corresponding control with each concentration-pretreated group of ketamine. Pefusates induced by ACh and high K+ were collected for 4 minutes, respectively. ∗∗p<0.01.
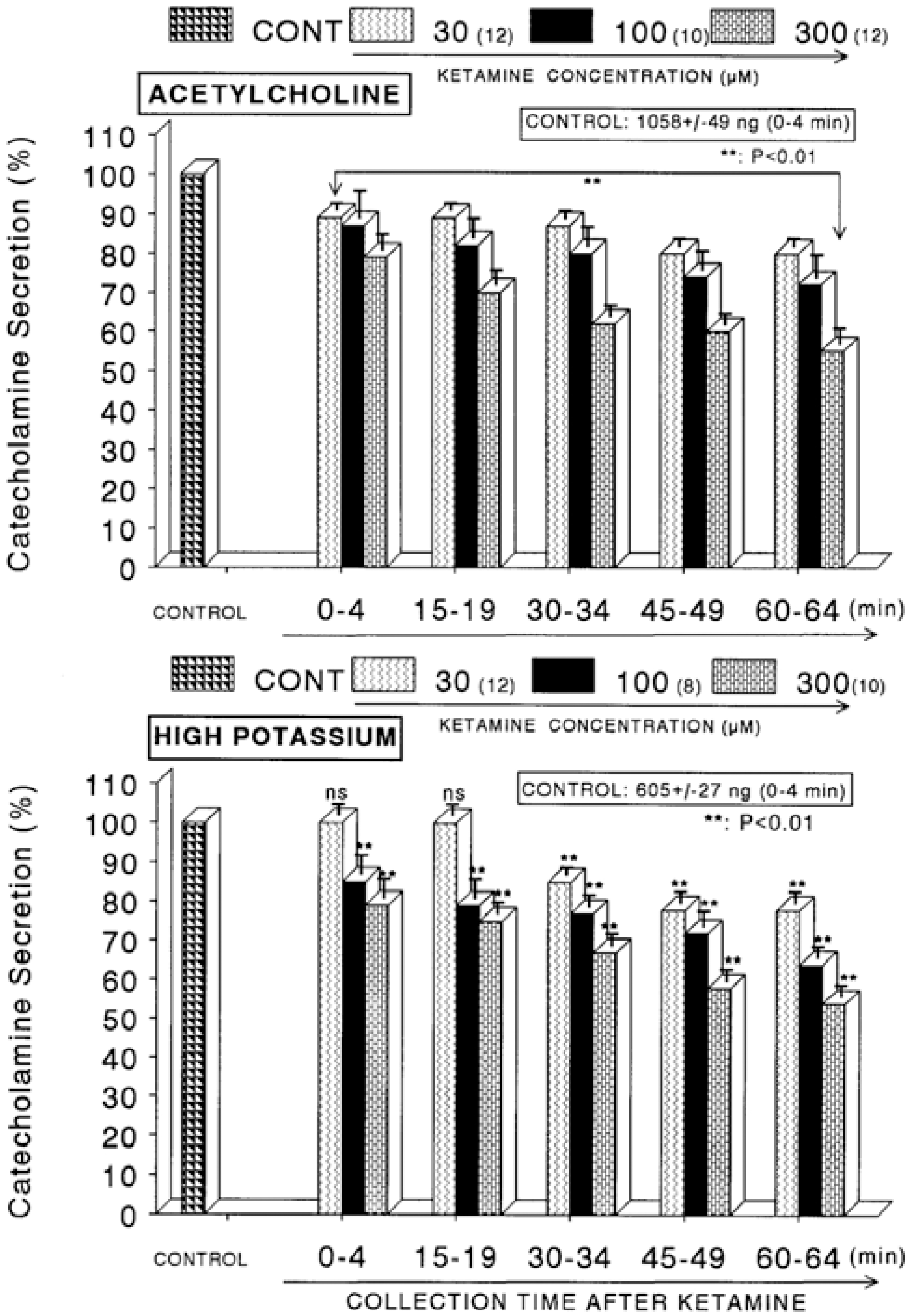
Fig. 2.
Concentration-dependent effects of ketamine on the CA secretory responses evoked by DMPP (Upper) and McN-A-343 (Lower) from the perfused rat adrenal glands. The CA secretory responses by the perfusion of DMPP (10−4 M) and McN-A-343 (10−4 M) for 4 min at 20 and 15 min intervals were induced after preloading with 30, 100 and 300μM of ketamine for 60 min, respectively. Pefusates induced by DMPP and McN-A-343 were collected for 8 and 4 minutes, respectively. Other legends are the same as in Fig. 1. ∗∗P<0.01. ns: Statistically not significant.
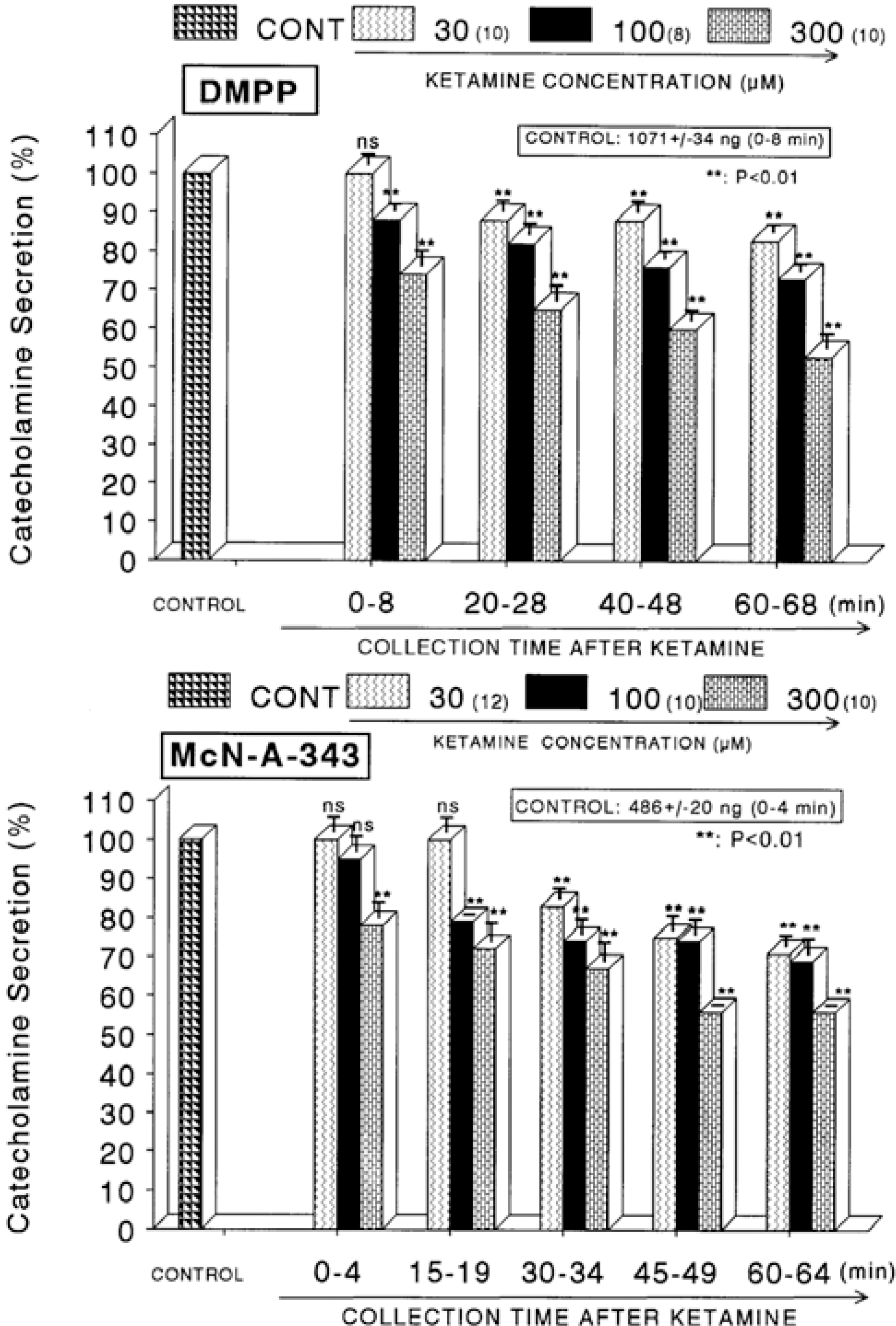
Fig. 3.
Time course effect of ketamine on the CA release evoked by Bay-K-8644 (Upper) and cyclopiazonic acid (Lower) from the perfused rat adrenal glands. Bay-K-8644 (10−5 M) and cyclopiazonic acid (10−5 M) were perfused into an adrenal vein for 4 min at 15 min intervals after preloading with 100μM of ketamine for 60 min, respectively. Other legends are the same as in Fig. 1. ∗P<0.05, ∗∗P<0.01. ns: Statistically not significant.
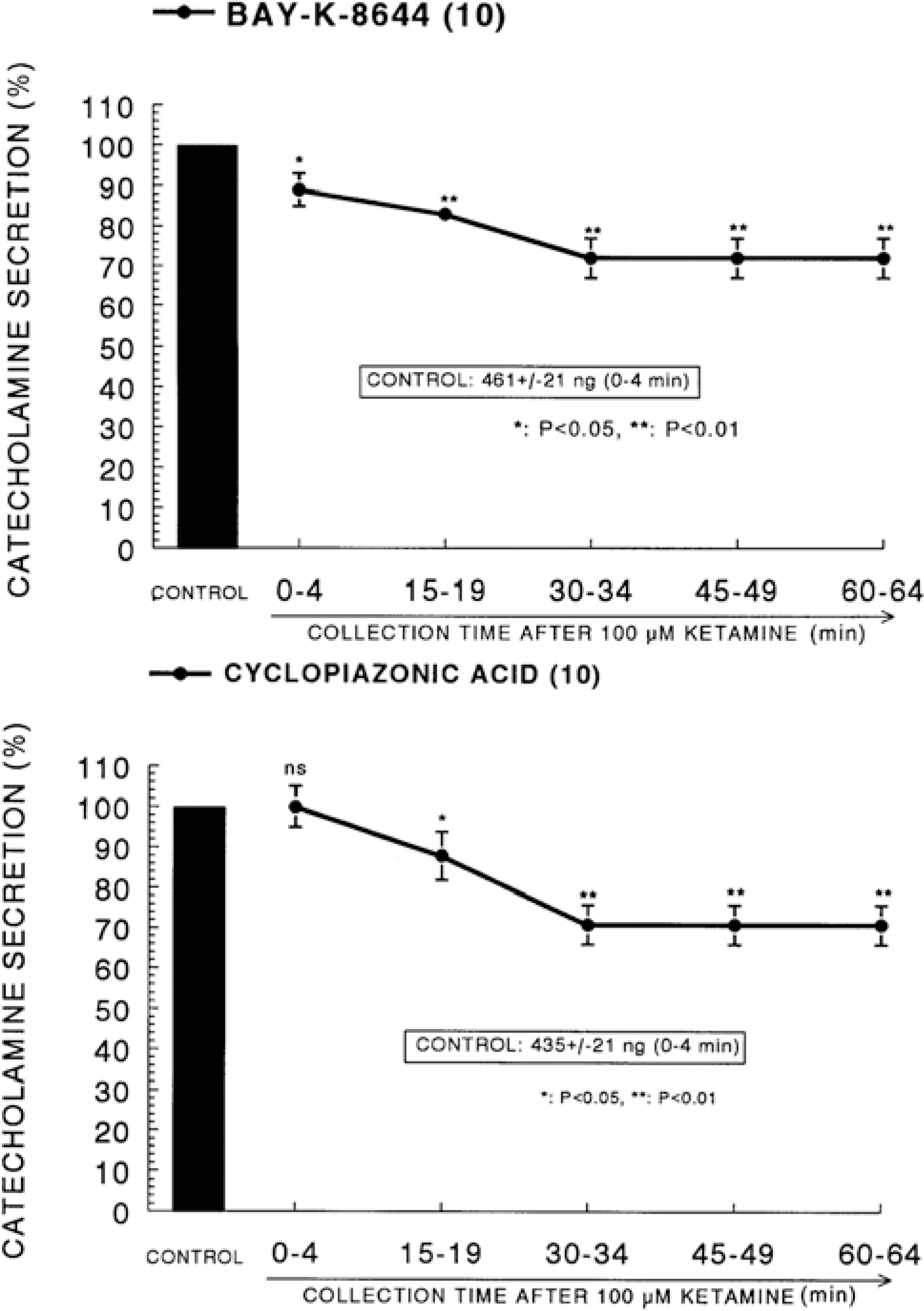
Fig. 4.
Time course effect of ketamine on the CA release evoked by veratridine from the perfused rat adrenal glands. Veratridine (10−4 M) was perfused into an adrenal vein for 4 min at 15 min intervals after preloading with 100μM of ketamine for 60 min. Other legends are the same as in Fig. 1. ∗∗P<0.01.
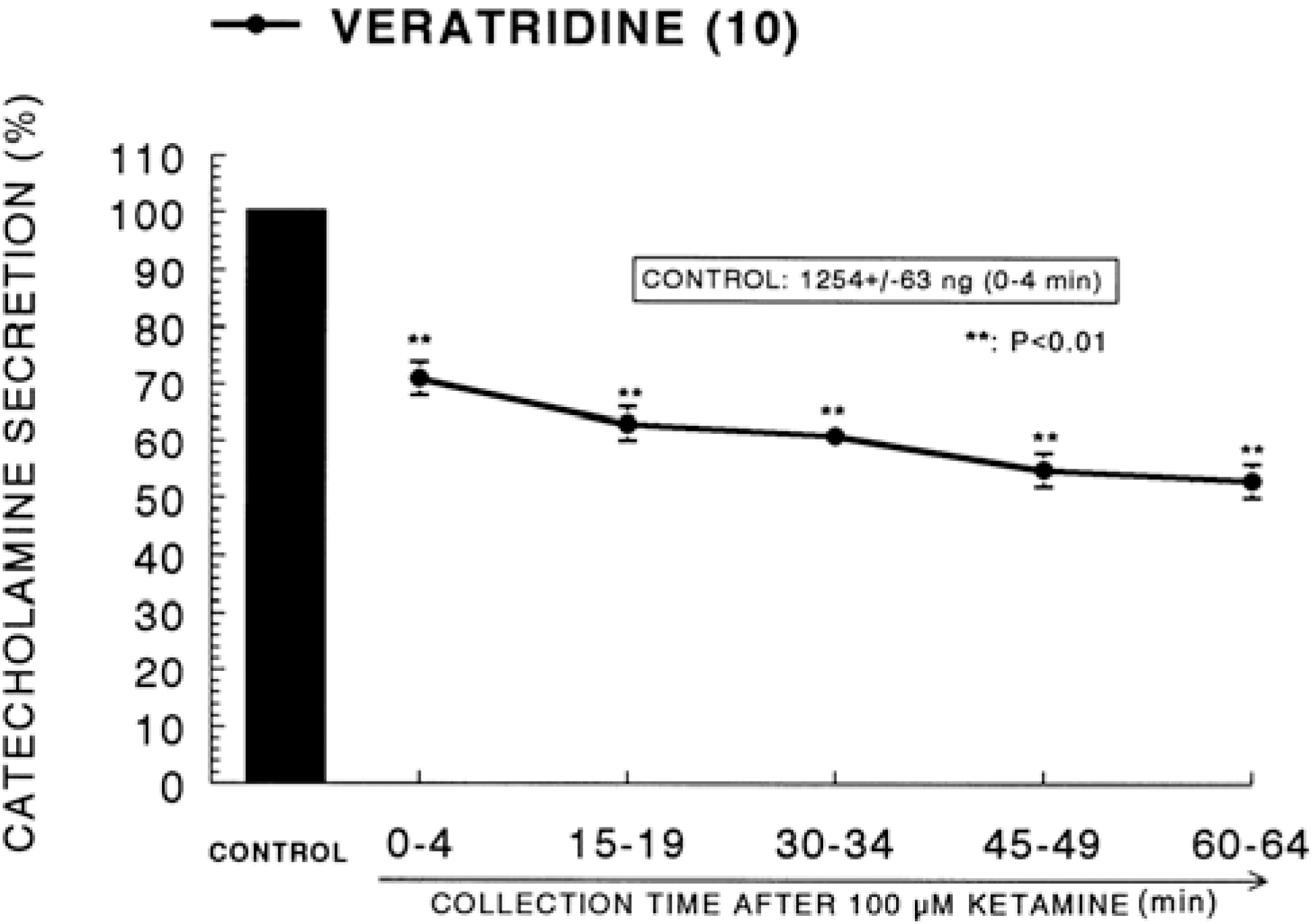
Fig. 5.
Time course effect of of thiopental on the secretory responses of catecholamines (CA) from the perfused rat adrenal glands evoked by acetylcholine (ACh, Upper) and by high K+ (Lower). CA secretion by a single injection of ACh (5.32×10−3 M) or K+ (56 mM) in a volume of 0.05 ml was evoked at 15 min intervals after preloading with 100 μM thiopentall for 60 min as indicated at an arrow mark. Other legends are the same as in Fig. 1. ∗∗P<0.01.
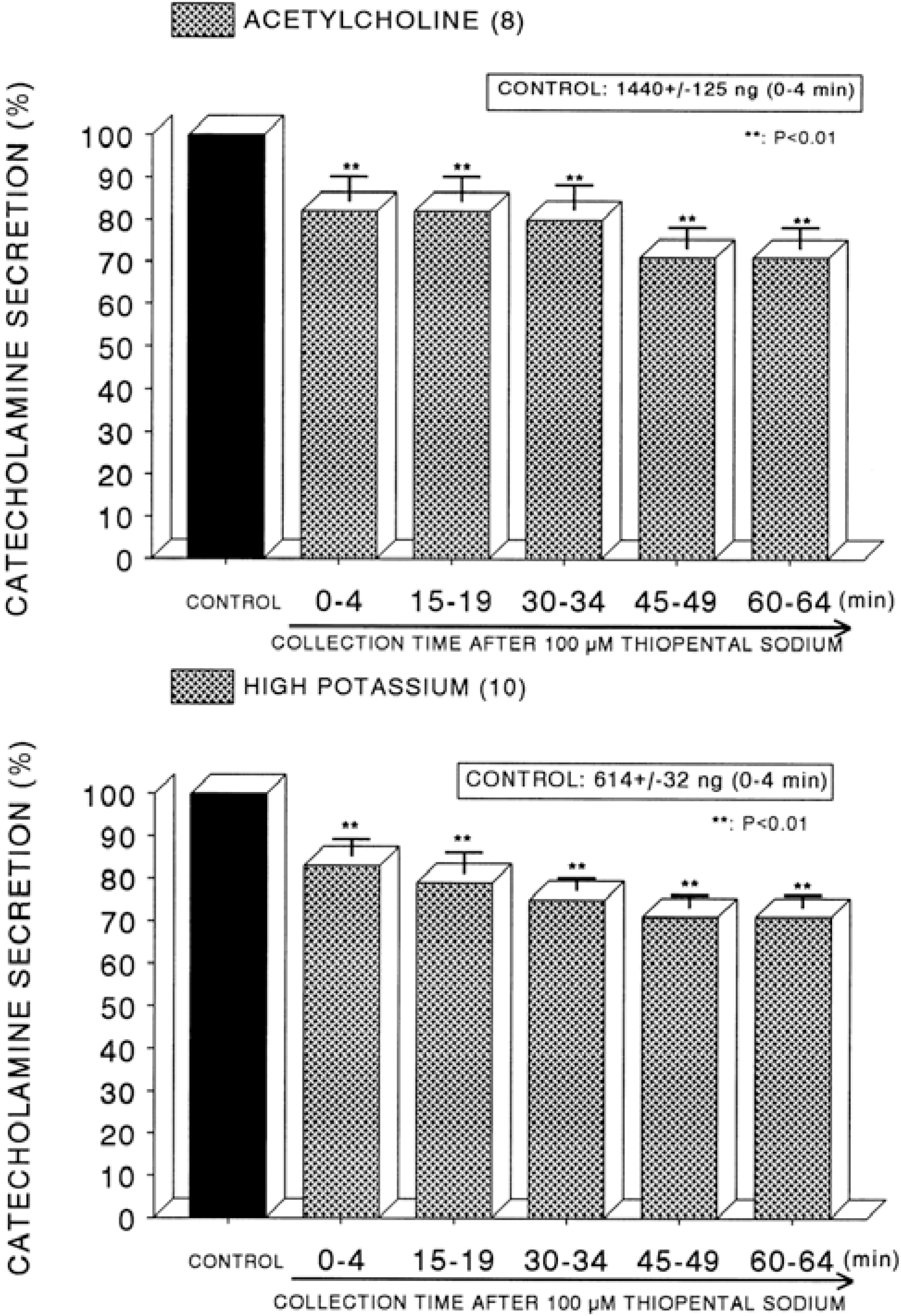
Fig. 6.
Time course effect of of thiopental on the CA secretory responses evoked by DMPP (Upper) and McN-A-343 (Lower) from the perfused rat adrenal glands. The CA secretory responses by the perfusion of DMPP (10−4 M) and McN-A-343 (10−4 M) for 4 min at 20 and 15 min intervals were induced after preloading with 100μM thiopental for 60 min, respectively. Other legends are the same as in Fig. 1. ∗∗P<0.01. ns: Statistically not significant.
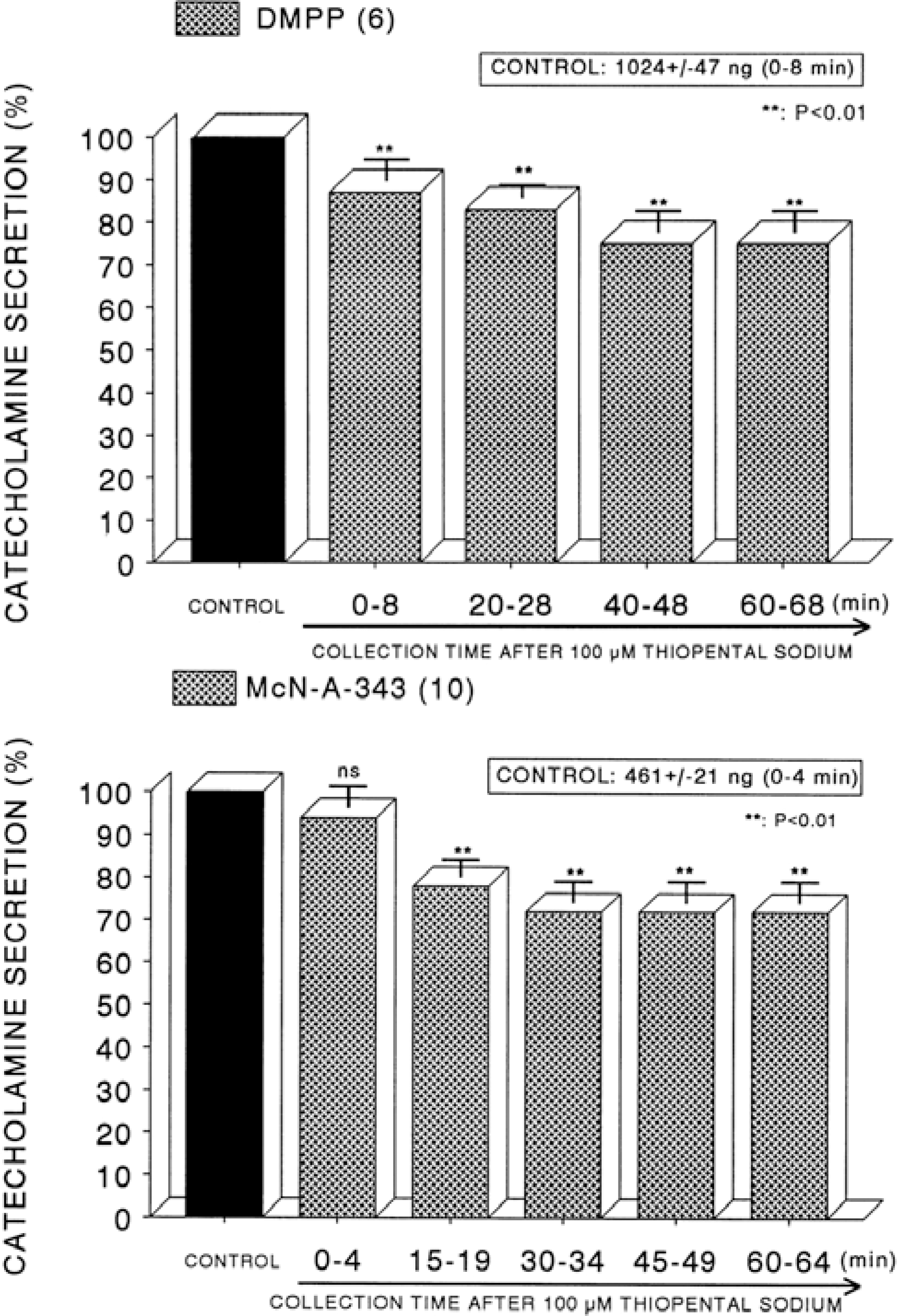
Fig. 7.
Time course effect of thiopental on the CA release evoked by Bay-K-8644 (Upper) and cyclopiazonic acid (Lower) from the perfused rat adrenal glands. Bay-K-8644 (10−5 M) and cyclopiazonic acid (10−5 M) were perfused into an adrenal vein for 4 min at 15 min intervals after preloading with 100μM of thiopental for 60 min, respectively. Other legends are the same as in Fig. 1. ∗P<0.05, ∗∗P<0.01. ns: Statistically not significant.
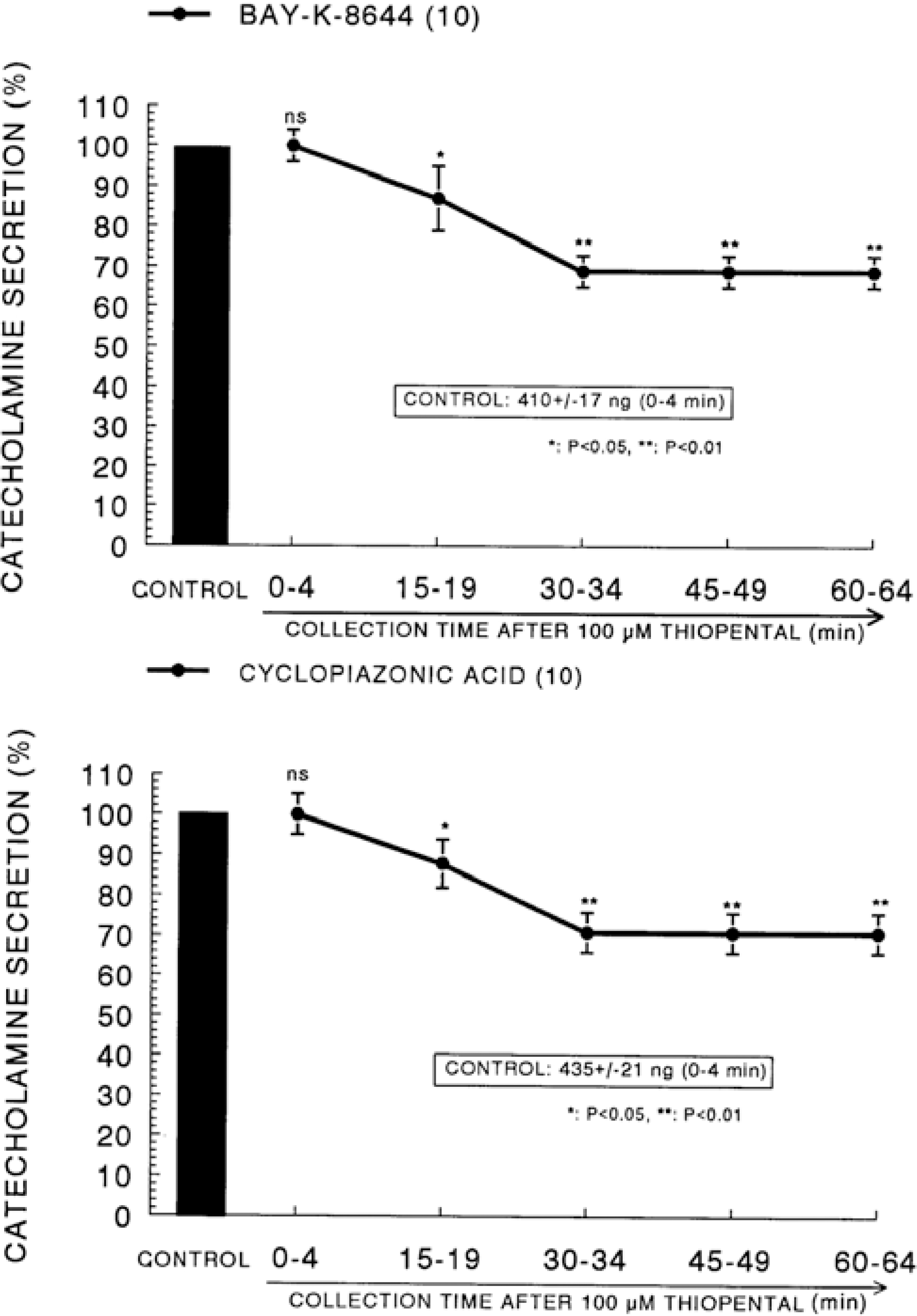
Fig. 8.
Time course effect of thiopental on the CA release evoked by veratridine from the perfused rat adrenal glands. Veratridine (10−4 M) was perfused into an adrenal vein for 4 min at 15 min intervals after preloading with 100μM of thiopental for 60 min. Other legends are the same as in Fig. 1. ∗∗P<0.01.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download