Abstract
Purpose
Our objective was to determine the effect of erythromycin (EM) in improving gastrointestinal motility in subtotal gastrectomized patients. We used radio-opaque Kolomarks as an objective method. We conducted a prospective, controlled clinical trial study of 24 patients.
Methods
All patients underwent subtotal gastrectomy with 3 capsules containing Kolomarks (20 markers per 1 capsule) in the remnant stomach before anastomosis. From the day of the operation to the 2nd postoperative day, patients in the EM group began receiving 200 mg of EM intravenously for 30 minutes continuously. We counted the number of Kolomarks in the stomach, passed by stomach, in rectum, and in stool with serial simple abdominal X-ray films on the first postoperative day up to the 7th postoperative day.
Results
The study population included 14 patients in the control group and 10 patients in the EM group. The two study groups were compared in terms of their characteristics including age, gender, past medical history, cancer stage, and operation type. No significant differences were found for the demographics between the two groups. We only found a significant difference for the number of Kolomarks passed by the stomach on the 3rd postoperative day (P = 0.026).
Postoperative ileus is an inevitable event after major abdominal surgery. It is caused by the cessation of the migrating motor complex (MMC), by hyperactivity of the sympathetic nervous system, by local inflammation secondary to surgery, and by the inhibitory effect of endogenous or exogenous opioids on gastrointestinal motility. Clinically, it is characterized by bowel distention, lack of bowel sounds, accumulation of gastrointestinal gas and fluid, and delayed passage of flatus and stool. Postoperative ileus can lead to cramp abdominal pain, nausea, vomiting, and potential complications including aspiration and acute gastric distention. When it is prolonged, postoperative ileus can lead to increased morbidity due to nutritional imbalance, length of hospital stay, and health-care cost. For these reasons, in particular, several pharmacological managements have been proposed [1].
Erythromycin (EM) is a well-known prokinetic agent. In patients with diabetic gastroparesis, EM, which stimulates the gastric antral and duodenal motilin receptor, significantly improves delayed gastric emptying of both solids and liquids [2]. It has also been shown to have potential benefits in patients with postvagotomy gastroparesis [3]. Although studies on the effects of EM in postoperative ileus are progressing these days, there have been only a few well designed clinical trials that have examined the use of EM in the postoperative setting [4-9].
The aim of this study was to determine the effect of EM for the improvement of gastrointestinal motility in subtotal gastrectomized patients. We used radio-opaque Kolomarks as the objective method. Therefore, we hypothesized that Kolomarks would pass through the gastrointestinal tract more rapidly in the EM group than in the control group.
The study was designed and implemented as a prospective, controlled clinical trial. From March 2007 to February 2008, all patients who were scheduled for subtotal gastrectomy due to gastric cancer at our institution were included in the study. The institutional review board approved this study, and each participant provided informed, written consent.
The patients were selected to one of two groups, either the control or EM group. Starting from the day of the operation to the 2nd postoperative day, patients in the EM group received intravenously 200 mg of EM infusion for 30 minutes in 100 mL of normal saline at 7:00 PM. In the control group, the patients were treated with conventional management without prokinetic agents. All patients during the observation period had their perioperative medications related to gastrointestinal motility stopped except for EM in the EM group.
All patients had stomach cancer. We classified the cancer staging using the American Joint Committee on Cancer, 8th edition. Subtotal gastrectomy with gastroduodenostomy (Billroth I) or gastrojejunostomy (Billroth II) was done by one surgeon at our institution. In addition, all patients had a truncal vagotomy done. At the last stage of the operation, we left 3 capsules (20 markers of Kolomarks per 1 capsule), for a total of 60 Kolomarks in the remnant stomach. During the operation, there were no complications and problems. We did not insert a nasogastric tube into patients during the postoperative period. Preoperative bowel preparation used one bottle of oral sodium biphosphate and one-half polyethylene glycol. Postoperative pain medications included patient-controlled analgesia with opioids and local analgesia administered by the anesthesiologist.
All patients in both groups had abdominal X-ray films taken on the 1st, 3rd, 5th, and 7th postoperative day. We counted the number of Kolomarks on each day. In addition, all patients had their passage of flatus checked daily by patient interview. We allowed all patients to have a meal after passage of flatus starting from sips of water to a soft diet in an incrementally manner each day. The length of the hospital stay was from the day of the operation to the discharge day since the patients' hospital stay for the preoperative evaluation varied. Glucose levels were measured on the 1st, 3rd, and 7th postoperative day in order to identify the correlation to bowel motility. We decided to discharge the patients, when the patients had a tolerable soft diet.
No adverse side effects potentially caused by EM were found in patients, which included nausea, emesis, abdominal pain, allergic reaction, and arrhythmia.
Comparisons between patients with and without EM with regards to demographics (age, gender), clinical parameters (past medical history, cancer stage, operation type, patients-controlled analgesia [PCA] categories), and outcomes (passage of flatus, lengths of hospital stay, glucose levels, number of Kolomarks) were carried out using chi-square test (nominal data), unpaired independent t-tests, and Mann-Whitney test. The Mann-Whitney test was used since the continuous parameters did not follow a normal distribution. Statistical analysis was done with SPSS ver. 18.0 (IBM, New York, NY, USA). A P-value < 0.05 was considered significant for all tests done.
Twenty-eight patients were enrolled in the study. Three patients with diabetes were excluded because we identified that the stimulation of antral motility by EM was attenuated by hyperglycemia [10]. In addition, another patient with esophageal cancer was ruled out due to a delayed hospital stay. The data from the 24 patients were used for the calculations in this study. The study population included 14 patients in the control group and 10 patients in the EM group. Comparisons between the two groups were done for age, gender, past medical history, cancer stage, operation type, and PCA categories. The average age for the control group and EM group was 54.4 ± 11.9 and 55.6 ± 11.8, respectively. Each group did not have any significant differences in operation type and cancer stage (Billroth I, 8 [57.1%] in control group vs. 6 [60%] in EM group; Billroth II, 6 [42.9%] in control group vs. 4 [40%] in EM group; stage I, II, III, 9 [64.3%], 5 [35.7%], 0 [0] in control group vs. 7 [70%], 1 [10%], 2 [20%] in EM group). However, no significant differences were found for the demographics between the two groups (Table 1).
The mean values for the glucose levels on the 1st, 3rd, and 7th postoperative day were higher in the EM group than in the control group, although there were no significant differences in each group. The mean values in the EM group for the passage of flatus and length of hospital stay were lower than in the control group. However, no significant differences were founded in each group (80.6 ± 23.8 in the control group vs. 78.8 ± 7.6 in the EM group; P = 0.744). Abdominal X-ray films were used to count the number of Kolomarks for each postoperative day (Figs. 1-4). On the 1st postoperative day, the mean value of the number of Kolomarks in the stomach was found to be higher in EM group than in the control group (54.8 ± 8.0 in the control group vs. 55.5 ± 4.9 in the EM group). The mean value of the number of Kolomarks passed by stomach on the 3rd postoperative day in the EM group was higher than in the control group (30.8 ± 25.6 in the control group vs. 49.9 ± 17.7 in the EM group). The mean values of the number of Kolomarks in the rectum on the 3rd postoperative day and in the stool on 5th and 7th postoperative day, also, were higher in the EM group than in the control group (0.4 ± 0.7 in the control group vs. 1.2 ± 1.6 in the EM group, 5.2 ± 12.4 in the control group vs. 10.5 ± 17.7 in the EM group, 19.9 ± 22.6 in the control group vs. 23.6 ± 25.1 in the EM group, respectively). However, we only found a significant difference for the number of Kolomarks passed by the stomach on the 3rd postoperative day (P = 0.026) (Table 2).
Motilin is a 22-amino acid that is secreted by the enterochromaffin cells of the duodenum and proximal small intestine. This hormone binds to high affinity motilin receptors that have been found in the stomach, the duodenum, and the colon [11]. Motilin receptor stimulation initiates the interdigestive (phase III) MMC. Return of an effective MMC is one of the important components for the resolution of postoperative ileus.
EM, discovered in 1952, was the first macrolide to be introduced into clinical practice [12]. It has been known for over 25 years that it acts as a motilin receptor agonist in the gut and gallbladder [13]. The intravenous form is the most potent stimulant of solid and liquid gastric emptying [14,15]. EM binds to motilin receptors and hence, increases the amplitude of antral peristalsis, triggers premature MMC phase III activity, and stimulates gastric emptying [16].
Different doses of EM may have different effects [2]. Forty milligrams of EM elicit premature phase 3 complexes that start in the stomach and migrate to the small intestine, while doses of 200 and 350 mg of EM elicit a burst of antral phase-3-like contractions that do not migrate to the small intestine, but are followed by a prolonged period of antral contractile activity. Motilin receptors are most dense in the stomach and decrease in density as they progress distally through the GI tract. Evidence suggests that motilin receptors are present in the colon but to a considerably lesser degree than in the remainder of the GI tract [11,17-19]. Consequently, the effect of EM on colonic motility and on the treatment of lower GI abnormalities has been inconsistent [20].
Interestingly, EM has also been shown to accelerate emptying in post-vagotomy and antrectomy patients [21]. This may be due to its stimulatory effects on the fundus. Vagus nerve activity appears to be more important in the stomach, where it promotes receptive relaxation of the fundus and contraction of the antrum, facilitating gastric emptying [22]. After vagotomy, emptying of liquids may be normal or accelerated, but emptying of solids is impaired. This can occur after peptic ulcer surgery but is more likely after gastric resection for malignancy or after inadvertent vagal nerve injury during antireflux surgery. In patients who underwent antrectomy and vagotomy, EM was shown to accelerate gastric emptying by roughly 40% as measured by solid-phase gastric emptying scintigraphy [15,21]. In a randomized controlled trial in 118 patients who underwent pancreaticoduodenectomy, intravenous EM reduced gastroparesis by 37% (measured by solid-phase gastric emptying study) and also reduced the need for nasogastric tube reinsertion [6].
In our study, we expected that the migration velocity of the Kolomarks passed by the stomach would accelerate in the EM group, although the patients had received a truncal vagotomy. As a result, we found that Kolomarks in the EM group were passed by the stomach more rapidly than in the control group on the 3rd postoperative day. There have been several studies on EM use postoperatively [4-6,8,9,23]. However, the studies did not prove exactly the effects of EM in the gastrointestinal tract postoperatively. Most of those studies used clinical parameters in order to obtain endpoints. Our study was more accurate than the other studies since the results of our study were calculated by an objective method (Kolomarks). However, our study also had limitations. Although we were able to distinguish between stomach gas and transverse colon gas, Kolomarks located in the transverse colon were confusing. In addition, there was some overlap when counting Kolomarks.
The pathogenesis of postoperative ileus is multifactorial. Therefore, we should approach the treatment of postoperative ileus diversely. Various medications have been used in an attempt to shorten the length of postoperative ileus after abdominal surgery, including antiemetics and prokinetics. Metoclopramide is an antiemetic and prokinetic agent that acts as a dopamine D2 receptor antagonist and mixed serotonin 5-HT3 antagonist/5-HT4 agonist. Metoclopramide also stimulates gastric emptying, as shown in controlled trials in patients in intensive care units [24]. The drug should not be used in patients with parkinsonism, due to its antidopamine properties. Neostigmine (Prostigmin) is a reversible acetylcholinesterase inhibitor that enhances the activity of neurotransmitter acetylcholine at muscarinic receptors. It is the first-line treatment for colonic ileus [25]. Alvimopan is an orally administered, peripherally acting antagonist for the µ-opioid receptor, which was developed as a potential treatment for postoperative ileus and opioid bowel dysfunction. In an early trial with 78 surgical patients, alvimopan demonstrated proof of principle that the µ-opioid receptor antagonistic activity shortened both postoperative ileus and hospital stay without compromising opioid-mediated analgesia [26].
In addition to previous mentioned pharmacological treatments, recently, methods that decrease local inflammation and opioids have been in the spotlight. Laparoscopic surgery has several advantages over open surgery. With smaller incisions, it is less traumatic to the body. The systemic inflammatory response appears to be less vigorous after laparoscopic surgery than after open surgery, as measured by circulating levels of interleukin (IL) 1, IL 6, and C-reactive protein [27]. Thoracic epidural analgesia has been shown to hasten the return of bowel function by 1 to 2 days and to reduce the need for opiates compared with systemic opioids alone [28].
We have demonstrated that intravenously infusion of 200 mg of EM in gastrorectomized patients induced rapid gastric emptying. However, the effect of EM is not enough for the entire gastrointestinal tract in postoperative patients. We expected that the recovery of the patients would be accelerated by proper nutritional support, and consequently, morbidity and length of hospital stay would decrease since the patients in the EM group after abdominal surgery had improved gastrointestinal motility. Although we identified the effect of EM in the stomach, we did not obtain satisfactory results for the passage of flatus and length of hospital stay and for the effect of EM in the small intestine and colon. We think that the results were influenced by doses and periods of application for EM as well as insufficient case number (total 24 patients), selection bias, and difficulties for counting Kolomarks. Therefore, even if we could use EM carefully for improvement of gastric emptying in postoperative patients, further studies with more randomized data number about doses and permissible periods of application for EM are needed to found the effect of EM in the entire gastrointestinal tract. Moreover, we should find a solution for postoperative ileus through studies on the exact impact of EM on the gastrointestinal tract as well as combination therapies.
In conclusion, our results have demonstrated that 200 mg of EM intravenously infusion during the postoperative period induced rapid gastric emptying, although it did not improve gastrointestinal motility for the entire gastrointestinal tract in subtotal gastrectomized patients.
Figures and Tables
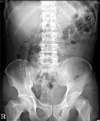
Fig. 1
Abdominal X-ray film in erythromycin group on 1st postoperative day. There are 57 Kolomarks in stomach.
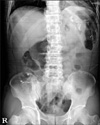
Fig. 2
Abdominal X-ray film in erythromycin group on 3rd postoperative day. All Kolomarks were passed by stomach.
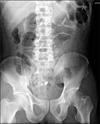
Fig. 3
Abdominal X-ray film in erythromycin group on 5th postoperative day. Picture shows that most Kolomarks in the gastrointestinal tract remained except 4.
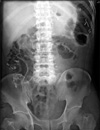
Fig. 4
Abdominal X-ray film in erythromycin group on 7th postoperative day. Twenty Kolomarks are remained in gastrointestinal tract.
ACKNOWLEDGEMENTS
We appreciate the good offices done by the writing center of Yonsei University. The authors thank Dr. Ho-Yong Yoon, Department of Surgery, Catholic Medical Center, Seoul, Korea for his help with data collection.
References
1. Maron DJ, Fry RD. New therapies in the treatment of postoperative ileus after gastrointestinal surgery. Am J Ther. 2008. 15:59–65.
2. Tack J, Janssens J, Vantrappen G, Peeters T, Annese V, Depoortere I, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992. 103:72–79.
3. Mozwecz H, Pavel D, Pitrak D, Orellana P, Schlesinger PK, Layden TJ. Erythromycin stearate as prokinetic agent in postvagotomy gastroparesis. Dig Dis Sci. 1990. 35:902–905.
4. Bonacini M, Quiason S, Reynolds M, Gaddis M, Pemberton B, Smith O. Effect of intravenous erythromycin on postoperative ileus. Am J Gastroenterol. 1993. 88:208–211.
5. Burt M, Scott A, Williard WC, Pommier R, Yeh S, Bains MS, et al. Erythromycin stimulates gastric emptying after esophagectomy with gastric replacement: a randomized clinical trial. J Thorac Cardiovasc Surg. 1996. 111:649–654.
6. Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993. 218:229–237.
7. Altomare DF, Rubini D, Pilot MA, Farese S, Rubini G, Rinaldi M, et al. Oral erythromycin improves gastrointestinal motility and transit after subtotal but not total gastrectomy for cancer. Br J Surg. 1997. 84:1017–1021.
8. Wilkinson NW, Gustafson RJ, Frizzi JD. The effect of erythromycin on bile excretion and proximal small bowel motility following divided gastric bypass surgery: a prospective randomized placebo-controlled trial. Obes Surg. 2002. 12:765–772.
9. Lightfoot AJ, Eno M, Kreder KJ, O'Donnell MA, Rao SS, Williams RD. Treatment of postoperative ileus after bowel surgery with low-dose intravenous erythromycin. Urology. 2007. 69:611–615.
10. Rayner CK, Su YC, Doran SM, Jones KL, Malbert CH, Horowitz M. The stimulation of antral motility by erythromycin is attenuated by hyperglycemia. Am J Gastroenterol. 2000. 95:2233–2241.
11. Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong SS, et al. Receptor for motilin identified in the human gastrointestinal system. Science. 1999. 284:2184–2188.
12. Mcguire JM, Bunch RL, Anderson RC, Boaz HE, Flynn EH, Powell HM, et al. Ilotycin, a new antibiotic. Schweiz Med Wochenschr. 1952. 82:1064–1065.
13. Itoh Z, Suzuki T, Nakaya M, Inoue M, Arai H, Wakabayashi K. Structure-activity relation among macrolide antibiotics in initiation of interdigestive migrating contractions in the canine gastrointestinal tract. Am J Physiol. 1985. 248(3 Pt 1):G320–G325.
14. DiBaise JK, Quigley EM. Efficacy of prolonged administration of intravenous erythromycin in an ambulatory setting as treatment of severe gastroparesis: one center's experience. J Clin Gastroenterol. 1999. 28:131–134.
15. Kendall BJ, Chakravarti A, Kendall E, Soykan I, McCallum RW. The effect of intravenous erythromycin on solid meal gastric emptying in patients with chronic symptomatic post-vagotomy-antrectomy gastroparesis. Aliment Pharmacol Ther. 1997. 11:381–385.
16. Richards RD, Davenport K, McCallum RW. The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol. 1993. 88:203–207.
17. Peeters TL. Erythromycin and other macrolides as prokinetic agents. Gastroenterology. 1993. 105:1886–1899.
18. Catnach SM, Fairclough PD. Erythromycin and the gut. Gut. 1992. 33:397–401.
19. Kaul A. Erythromycin as a prokinetic agent. J Pediatr Gastroenterol Nutr. 2002. 34:13–15.
20. Longo WE, Vernava AM 3rd. Prokinetic agents for lower gastrointestinal motility disorders. Dis Colon Rectum. 1993. 36:696–708.
21. Ramirez B, Eaker EY, Drane WE, Hocking MP, Sninsky CA. Erythromycin enhances gastric emptying in patients with gastroparesis after vagotomy and antrectomy. Dig Dis Sci. 1994. 39:2295–2300.
22. Johnson MD, Walsh RM. Current therapies to shorten postoperative ileus. Cleve Clin J Med. 2009. 76:641–648.
23. Smith AJ, Nissan A, Lanouette NM, Shi W, Guillem JG, Wong WD, et al. Prokinetic effect of erythromycin after colorectal surgery: randomized, placebo-controlled, double-blind study. Dis Colon Rectum. 2000. 43:333–337.
24. Jooste CA, Mustoe J, Collee G. Metoclopramide improves gastric motility in critically ill patients. Intensive Care Med. 1999. 25:464–468.
25. De Giorgio R, Knowles CH. Acute colonic pseudo-obstruction. Br J Surg. 2009. 96:229–239.
26. Taguchi A, Sharma N, Saleem RM, Sessler DI, Carpenter RL, Seyedsadr M, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001. 345:935–940.
27. Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: A prospective randomized trial. Ann Surg. 2000. 231:506–511.
28. Zingg U, Miskovic D, Hamel CT, Erni L, Oertli D, Metzger U. Influence of thoracic epidural analgesia on postoperative pain relief and ileus after laparoscopic colorectal resection: Benefit with epidural analgesia. Surg Endosc. 2009. 23:276–282.




 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download