Abstract
Although the majority of forequarter amputations are performed for high-grade bone and soft tissue sarcomas or extensive osteomyelitis of the upper extremity, this radical operation may also be indicated for the curative treatment of recurrent breast cancer and for the palliation of locally advanced breast cancer. We report a male patient with metastatic breast adenocarcinoma who underwent simultaneous mastectomy and forequarter amputation for the management of both his primary and metastatic disease.
Breast carcinoma in men is uncommon with an incidence report rate of 1.08 per 100,000 [1]. At time of diagnosis, men have a higher median age; and are more likely to have a more advanced stage of disease and lymph node involvement [1].
Forequarter (interscapulothoracic) amputation was first performed in 1808 for severe traumatic injuries of the upper extremity. The procedure involves removal of the entire upper extremity with the ipsilateral scapula and total/ subtotal clavicle. The first oncological forequarter amputation was reported in 1836 [2]. Since then, it has been used for managing high-grade bone and soft-tissue sarcomas of the shoulder girdle in which salvage of the extremity and the aforementioned bones including the shoulder joint would not be feasible. Today, it is very rarely used as effective adjuvant chemotherapy with or without radiation therapy combined with limb-sparing surgery in many cases has replaced radical amputation surgeries [3].
Although the majority of forequarter amputations are performed for high-grade bone and soft tissue sarcomas or extensive osteomyelitis of the upper extremities, this radical operation may also be indicated for the curative treatment of recurrent breast cancer and for the palliation of locally advanced breast cancer [4]. Axillary tumor recurrence may cause significant morbidity due to pain, limb dysfunction, lymphedema, and varying degrees of paralysis and sensory impairment [3,4]. It may also cause chest wall invasion, and skin ulceration secondary to invasion of the brachial plexus and axillary vessels by the tumor [3,4]. Further morbidity can be caused by perforation of the overlying skin by the tumor causing bacterial or fungal infections, sepsis, or hemorrhage. In those cases with dysfunctional upper extremities, a need for a more radical surgery may arise in order to improve the patient's quality of life and daily function [3,5-7].
To date, there have only been 20 published cases of forequarter amputations for axillary recurrence of breast cancer. All of the cases were females and only 4 of these amputations were performed primarily for curative purposes [4]. We are hereby reporting a male patient with metastatic breast adenocarcinoma who underwent simultaneous modified radical mastectomy and forequarter amputation.
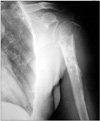
A 54-year-old male presented with masses on his left arm and axilla. The axillar mass was 12 × 7 cm and the mass in the proximal lateral humerus was 6 × 5 cm. His symptoms had started a number of years prior. Before the initiation of these symptoms, he denied having any significant medical problems. He had a history of smoking for 20 years. In his family history, no relevant medical condition and/or history of cancer was identified. He had been referred to a surgerical clinic three years prior in which he had a biopsy of the tumor reported as mucinous adenocarcinoma. It appears that he had been evaluated only for possible intestinal primaries at that time and having failed to identify the primary, had been sent to receive radiotherapy of unclear dosage. After the recurrence of his tumor, he was referred to the medical oncology department at our hospital with a mass in the left thoracic region and another in the left humerus, visible on X-rays (Fig. 1). His examination revealed a mass in his left breast besides the aforementioned ones. His biopsy materials were reevaluated in our pathology department and confirmed to be metastatic adenocarcinoma. The report also indicated that the mass might be breast originated, raising the suspicion towards a possible breast primary. He was then given neoadjuvant chemotherapy consisting of docetaxel and capecitabine for six cycles following the decision of our tumor council.
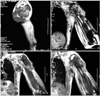
An magnetic resonance imaging (MRI) scan was obtained following the cessation of his chemotherapy revealing a 140 × 100 × 90 mm lobulated mass in the proximal epiphyses, metaphysis and diaphysis of the left humerus, which was destructing the cortices on multiple sides with a large soft tissue component, and invading and protruding out of the skin (Fig. 2).
Another mass, 123 × 54 × 74 mm in size, was located in the axillary region adjacent to the thoracic wall infiltrating the skin, surrounded by multiple nodular masses of different sizes. These masses also invaded the brachial plexus and the vessels. There were other masses also in his pectoral muscle, as well as in his scapular muscles. Medullar intensity of the scapula appeared to be normal. Computed tomography scans of the chest, abdomen and pelvis and an MRI of the brain failed to demonstrate any other metastasic lesions.
At the end of the neoadjuvant chemotherapy protocol, the patient was in intractable pain. He was under a combination analgesic treatment that consisted of ibuprofen (3 × 400 mg), tramadol (4 × 50 mg) and pethidine (1 × 75 mg). His pain scales (out of 10) were 8 to 9 in the mornings and 9 to 10 at night, severe enough to awaken him from his sleep. The status of the patient was discussed amongst the tumor council of our hospital consisting of orthopedic surgeons, radiologists, medical oncologists and radiation oncologists, who decided that amputation would be the best option for the management of the patient's pain and impaired function.
The radical nature of the procedure as well as the risks and benefits were discussed with the patient and his family and the patient consented to undergo the operation. The operation was performed by a team of orthopedic, general and plastic-reconstructive surgeons.
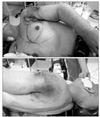
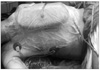
He was intubated and placed in a right lateral decubitus position. The skin incision was marked in close consultation with the general and plastic surgeons (Fig. 3).
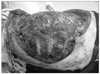
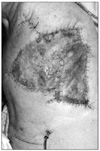
Skins flap were elevated superiorly to the clavicle, inferiorly to the costal arch, and medially to anterior midline. The lateral border of the skin flaps was latissimus dorsi muscle, inferiorly; and the supero-medial border of the mass, superiorly. Dissection of the pectoralis major muscle was started from the medial side at the origin from the sternum as the insertion was also invaded by the mass. Dissection was then directed to the thoracic wall to excise all the breast tissue, pectoral muscles and the soft tissues, including the periosteum of the ribs without exposing the tumor. The subclavian vessels were ligated and divided. The exposure was continued laterally and posteriorly up to the insertions of rhomboid muscles. Finally, the upper extremity, all together with the mastectomy material, was removed including the scapula, inevitably creating a big tissue defect (Fig. 4). The defect was closed with full thickness skin graft and application of vacuum assisted closure (V.A.C. Therapy, KCI Inc., San Antonio, TX, USA) (Fig. 5).
The histopathological observation of the amputation material was then carried out. His biopsy from the excised mass revealed infiltrative ductal carcinoma with negative surgical margins. The specimen consisted of 70 cm of upper extremity and scapula and breast tissue attached to it. Multiple nodules were detected and the biggest nodule was 13 cm in diameter. The axillar region was completely infiltrated with tumor and ulcerated. There was another ulcerated region of 6 × 5 cm in the lateral proximal humeral region. There was another nodule of 0.5 cm in the medial side of the elbow. The tumor was negative for estrogen receptor, c-erbB2 and GCDFP-15, and 1% positive for progesterone receptor. In Fluorescence in situ Hybridization (FISH) studies conducted with DNA probes (REPEAT-FREE POSEIDON FISH DNA Probes, Kreatech Diagnostics, Amsterdam, Netherland) HER-2 gene amplification was not manifested.
The patient was monitored in the post anesthesia care unit for a few days until he was stabilized and then transferred to our clinic. In the early post-operative period his pain medication was ibuprofen (3 × 400 mg) and tramadol (2 × 50 mg), with the addition of morphine patient controlled analgesia for the first two days. His pain scales, out of 10, were between 4 to 6 both during the day and at night. He was discharged from the hospital when the wound healed after two weeks (Fig. 6). His outpatient progress was monitored during the first, third, sixth and twelfth weeks post-operatively. In the third and the sixth week controls, the patient reported that he was experiencing reduced levels of pain and had therefore lessened his intake of pain medication; he was only taking ibuprofen (3 × 400 mg). In the control during the twelfth week the patient reported that he was almost entirely pain-free. Unfortunately, a lung metastasis was then discovered at six months. The patient, refusing to receive any further chemotherapy, passed away eleven months after surgery.
Invasive breast cancer is the most common malignancies among women [8]. Although the incidence appears to be on the rise, due to earlier detection and adjuvant therapy, declining mortality rates have been reported [9]. With the emergence of newer techniques, there is a tendency for limited axillary surgery, which may eventually increase the potential risk of future axillary recurrences [8]. A review of the literature reveals only 20 published cases of forequarter amputation for axillary recurrence of breast cancer, most derived from small groups or individual case series [4]. None of these patients were men, and to our knowledge, forequarter amputation as a primary treatment for metastatic and infiltrative breast cancer has never been reported.
The patient described in this study was handled via a multidisciplinary approach. The status of the disease and possible treatment alternatives were discussed amongst the tumor council at our hospital, which consists of orthopedic surgeons, radiologists, medical oncologists and radiation oncologists. The operation of simultaneous mastectomy, forequarter amputation and skin grafting was performed by a team of orthopedic, general and plastic-reconstructive surgeons.
It was well known at the time of treatment that our patient would have a poor short-term prognosis with his extensive metastasis, and the operation was performed with palliative aim, focusing on his quality of life rather than life expectancy. Eventually his quality of life improved dramatically after the operation and he lived for eleven more months without suffering from severe pain. Similarly, an overall improvement in daily life, physical and emotional well-being and sexual or social life is reported for patients who underwent forequarter amputation for palliation [5,7]. Furthermore, it has also been advocated that local recurrence of breast cancer without a remote metastasis can be an indication for surgery to relieve pain and bleeding, and that in combination with adjuvant therapy the patient's survival can be prolonged [10].
In conclusion, this case demonstrates that even in grossly advanced stages of breast carcinomas with invasion not only to the chest wall but also to the neighboring axilla and extremity, ablative surgery of a very large magnitude including the excision of the breast and all chest wall muscles as well as the entire shoulder girdle and upper extremity, is not only feasible with a multidisciplinary approach but also advisable in order to decrease patients' pain and improve the quality of their lives.
Figures and Tables
References
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004. 101:51–57.
2. Yoak MB, Cocke WM Jr, Carey JP. Interscapulothoracic amputation. W V Med J. 2001. 97:148–150.
3. Wittig JC, Bickels J, Kollender Y, Kellar-Graney KL, Meller I, Malawer MM. Palliative forequarter amputation for metastatic carcinoma to the shoulder girdle region: indications, preoperative evaluation, surgical technique, and results. J Surg Oncol. 2001. 77:105–113.
4. Goodman MD, McIntyre B, Shaughnessy EA, Lowy AM, Ahmad SA. Forequarter amputation for recurrent breast cancer: a case report and review of the literature. J Surg Oncol. 2005. 92:134–141.
5. Merimsky O, Kollender Y, Inbar M, Lev-Chelouche D, Gutman M, Issakov J, et al. Is forequarter amputation justified for palliation of intractable cancer symptoms? Oncology. 2001. 60:55–59.
6. Malawer MM, Buch RG, Thompson WE, Sugarbaker PH. Major amputations done with palliative intent in the treatment of local bony complications associated with advanced cancer. J Surg Oncol. 1991. 47:121–130.
7. Rickelt J, Hoekstra H, van Coevorden F, de Vreeze R, Verhoef C, van Geel AN. Forequarter amputation for malignancy. Br J Surg. 2009. 96:792–798.
8. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003. 53:5–26.
9. Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000. 355:1822.
10. Hanagiri T, Nozoe T, Yoshimatsu T, Mizukami M, Ichiki Y, Sugaya M, et al. Surgical treatment for chest wall invasion due to the local recurrence of breast cancer. Breast Cancer. 2008. 15:298–302.




 Citation
Citation Print
Print


 XML Download
XML Download