Abstract
The epidermis contains epithelial cells, immune cells, and microbes which provides a physical and functional barrier to the protection of human skin. It plays critical roles in preventing environmental allergen penetration into the human body and responsing to microbial pathogens. Atopic dermatitis (AD) is the most common, complex chronic inflammatory skin disease. Skin barrier dysfunction is the initial step in the development of AD. Multiple factors, including immune dysregulation, filaggrin mutations, deficiency of antimicrobial peptides, and skin dysbiosis contribute to skin barrier defects. In the initial phase of AD, treatment with moisturizers improves skin barrier function and prevents the development of AD. With the progression of AD, effective topical and systemic therapies are needed to reduce immune pathway activation and general inflammation. Targeted microbiome therapy is also being developed to correct skin dysbiosis associated with AD. Improved identification and characterization of AD phenotypes and endotypes are required to optimize the precision medicine approach to AD.
Atopic dermatitis (AD) is the most common chronic skin disease worldwide.12 It affects about 20% of children and 5% of adults.1345 Patients with persistent or severe AD suffer from profound impairment of their quality of life.267 Additionally, AD places a heavy economic burden on patients and their family.89 AD is strongly associated with the development of food allergy, bronchial asthma, and allergic rhinitis, commonly referred to as the Atopic March.101112131415 The epidermis provides a physical and functional barrier to the human body, and skin barrier defects are the most important pathologic findings in AD skin.161718 Skin barrier defects have been considered an initial step in developing AD.417 Recently, investigators have demonstrated that multiple factors, including immune dysregulation, defects in terminal epithelial differentiation such as lack of filaggrin (FLG), deficiency of antimicrobial peptides (AMPs), altered composition of stratum corneum intercellular lipids, and altered skin microbiome may affect skin barrier function (Fig. 1).24161920 These factors interact with each other and may modify skin barrier function. In this review, we discuss normal skin barrier and pathogenesis of skin barrier defects associated with the development of AD skin disease. Additionally, we review the role of emollients, anti-inflammatory agents, sodium hypochlorite, probiotics, and microbiome in the treatment and prevention of AD development. Moreover, various types of immune-directed targets for biologic therapy are reviewed.
The skin barrier plays a critical role in preventing allergen and microbial penetration into the human body.41021 The epidermis consists of a 15- to 30-nm-thick layer of proteins and lipids, and provides a physical and functional barrier to the human body.2223 The physical skin barrier is mainly localized to the uppermost area of the epidermis which is the cornified layer (stratum corneum).2224 The epidermis is continuously regenerated by terminally differentiating keratinocytes, which is known as cornification or keratinization.2223 Cornification begins with the migration of keratinocytes from the basal to upper layers, and ends with the formation of the cornified layer.2223 During epidermal differentiation, lipids are produced by keratinocytes and extruded into the extracellular space to form extracellular lipid-enriched layers.222324 Omega-hydroxy-ceramides are covalently bound to cornified envelope proteins and form the backbone for the subsequent addition of free ceramides, free fatty acids, and cholesterol in the cornified layer.222324 The epidermis undergoes complete turnover every 28 days.25
Cell proliferation, differentiation, and death occur sequentially, and each process is characterized by the expression specific proteins, including occludin, claudins, keratins, transglutaminases (TGs), loricrin, and FLG.22232627 Keratinocytes express specific differentiation markers according to their stage of epidermal differentiation.22 For instance, keratin 5 and TG2, which are expressed in the basal layer, represent early differentiation markers. In contrast, FLG, which is expressed in the upper granular and cornified layers, is a late differentiation marker. Tight junctions (TJs), desmosomes, and adherens junctions are paracellular proteins that form a permeability barrier between adjacent cells and involve cell adhesion.26272829
Keratinocytes also produce AMPs including cathelicidin (LL-37) and beta-defensins (HBDs), which kill microbes and play important roles in maintaining skin homeostasis.3031 In addition to their antibacterial activity, AMPs kill viruses and fungi through multiple modes of action.31 The levels of AMPs, such as HBDs and LL-37 in epidermis, are low in normal health conditions, but are highly expressed upon infection and inflammation.3132 AMPs form an innate epithelial chemical barrier and have pleiotropic functions.3133 They not only kill microbes, but also control inflammation and regulate the skin barrier.313435 Impaired TJ protein expression contributes to skin barrier dysfunction in AD.36 HBD-3 improves the function of the epithelial TJ barrier by inducing expression of several claudins.34 HBDs and LL-37 also induce production of IL-18 through p38 and Erk mitogen-activated protein kinase activation in human keratinocytes.37 Additionally, they induce expression of IL-6, IL-10, macrophage inflammatory protein-3 alpha, and RANTES.38 Furthermore, it has been reported that HBDs and LL-37 induce keratinocyte migration, proliferation, re-epithelialization, neovascularization, and wound healing.31353839
The cornified layer is surrounded by a continuous lipid matrix which provides a barrier against water and prevent water loss.244041 The lipid matrix mainly consists of 3 lipid classes: cholesterol, free fatty acids, and ceramides.2342 Therefore, the lipid matrix in the cornified layer may play crucial roles as a part of skin barrier and shows altered composition in AD skin.
It has also recently been reported that the epidermal microbiome may also play crucial roles in maintaining skin barrier function.41643 Previously, the biogeography of the skin microbiome has been reported in children and adults.4445 Several studies have shown that human skin microbiome is site-specific.444546 Recently, it has been reported that the gut and cutaneous commensal bacteria, including Staphylococcus (S.) epidermidis, and S. hominis, play important roles in skin homeostasis and host defense against microbial penetration.47484950
Epidermal barrier proteins, including FLG, TGs, keratins, loricrin and intercellular proteins, are cross-linked to form an impermeable skin barrier.2223 Skin barrier defects facilitate allergen sensitization and lead to systemic allergic responses, such as increased IgE levels and airway hyperreactivity.36515253 Transepidermal water loss (TEWL) is a noninvasive measurement used to evaluate skin barrier function.54 Patients with AD have increased TEWL, which reflects skin barrier dysfunction in AD, and can precede clinical AD.5556
AD skin is characterized by overexpression of Th2 and Th22 cytokines that contribute to skin barrier dysfunction by altering protein and lipid content in the skin (Table 1).245758 FLG is a key epidermal barrier protein.2259 It is degraded into free amino acids and these amino acids are essential for maintaining skin pH and the retention of water contributing to osmolarity in the cornified layer.606162 FLG deficiency alters the shape of corneocytes in the skin and enhances skin inflammation by facilitating epicutaneous sensitization in murine models of eczema.4163 FLG deficiency also causes paracellular skin barrier abnormality that reduces inflammatory thresholds to irritants and haptens.4164 FLG proteolysis occurs upon exposure to a low humidity environment and can be prevented by high humidity.65 FLG is decreased in AD skin by overexpression of IL-4, IL-13, IL-25, IL-17A, and IL-22 (Fig. 2).57596667 Additionally, loricrin, and involucrin, which are major epidermal barrier proteins, are also down-regulated by Th2 cytokines through STAT6 signaling in AD skin.68 It is well known that FLG mutation is a major predisposing factor for AD development, particularly in patients who have early-onset AD and those with persistent AD.215569707172 However, a significant number of AD patients do not have any type of FLG gene mutation, and about 40% of individuals with FLG-null alleles do not have AD.2173 Moreover, most of the patients with AD and FLG mutations eventually recover from AD.217374 Therefore, FLG mutations contribute to AD, but in isolation it is not sufficient to generate AD. There are other factors that result in AD development. Intercellular proteins, including TJs, desmosomes, and adherens junctions, form a permeability barrier between adjacent cells and aids with cell adhesion.26272829 Th2 cytokines down-regulate TJs, and impaired TJs contribute to abnormal skin barrier function in AD.3675 Corneodesmosin (CDSN) is an intercellular protein that plays a critical role in maintaining skin barrier function.2976 Recently, Lee et al.77 have reported that CDSN expression is down-regulated by cytokines, including IL-4, IL-13, IL-22, IL-25, and IL-31. Additionally, CDSN deficiency resulted in lethal-skin barrier disruption in a mouse model,76 and enhanced viral penetration in an organotypic skin model.77 Therefore, a variety of cytokines modulate epidermal barrier proteins, and cause skin barrier defects.
AMPs, such as LL-37 and HBD-3, are highly expressed by keratinocytes during infection, inflammation, and wounding.3031 AMPs form an innate multidimensional epithelial chemical barrier.3133 They not only have antimicrobial activities, but also regulate the skin barrier.313435 AMP expressions are inhibited in AD skin by Th2 cytokines, which are overexpressed in AD skin.32787980 The deficiency of AMPs and over-expressed Th2 cytokines in AD skin is associated with a higher propensity to S. aureus infection, which is known to play critical roles in the exacerbation of AD.303181 Son et al.82 have reported that S. aureus inhibited expression of terminal differentiation markers, including FLG, loricrin, and keratina 1 and 10. Recently, Brauweiler et al.83 have also demonstrated that S. aureus lipoteichoic acid inhibits keratinocyte differentiation markers, including keratins 1 and 10, and desmocollin1, through a p63-mediated pathway. Therefore, deficiency of AMPs and overexpressed Th2 cytokines in AD skin may lead to frequent microbial skin infections and skin barrier defects.328485
AD skin also has a defective lipid matrix. This causes impaired skin barrier function.188687 Stratum corneum intercellular lipid composition in AD skin is characterized by altered expression of enzymes involved in the biosynthesis of free fatty acids and ceramides.8688 Researchers have demonstrated that altered composition of stratum corneum intercellular lipids correlates with S. aureus colonization status in AD.89 Additionally, it has been reported that a synthetic omega-hydroxyceramides enhanced the integrity of the stratum corneum, and accelerated the recovery of damaged skin barrier function by stimulating differentiation processes.90 Lowe et al.91 also reported that routine lipid replacement reduced the incidence of AD during the active treatment period by approximately fifty percent. Therefore, the lipid matrix in the cornified layer may play a crucial role as part of the skin barrier.
AD is associated with abnormal skin colonization of pathogens, such as S. aureus.492 Commensal bacteria induce AMPs and inhibit S. aureus on the human skin.16 In contrast, cutaneous dysbiosis affects skin immune responses and causes skin inflammation.499394 Moreover, skin dysbiosis may cause skin barrier defects.9596 Species-level investigation of AD flares demonstrated greater S. aureus predominance in patients with more severe disease, and S. epidermidis predominates in patients with less severe disease.49 Additionally, S. aureus isolates from AD patients with more severe flares induced epidermal thickening and expansion of cutaneous Th2 and Th17 cells.49 However, Kennedy et al.97 reported that commensal staphylococci were significantly less abundant in infants with AD. This finding suggests that commensal bacteria might protect against the development of AD.
AMPs, such as HBD-3 and LL-37, are highly expressed after various exposures in the normal healthy skin.31 Down-regulated AMPs by Th2 cytokines in AD skin causes recurrent microbial infections and may affect skin pH.418598 Several factors, including FLG, cytokines, proteases, enzymes, and microbes, alter skin pH.429899 Skin pH is an important factor controlling skin homeostasis. Increased skin pH also facilitates microbial skin infections and skin barrier defects.49899 Additionally, Brauweiler et al.100 have demonstrated that staphylococcal alpha toxin, a primary toxin of S. aureus, causes cell death and consequently skin barrier defects. Thus, decreased levels of AMPs may cause skin dysbiosis and skin barrier defects. In summary, the skin dysbiosis and deficiency of AMPs may affect skin homeostasis and cause skin barrier defects in AD skin.4164749 However, additional studies are needed to elucidate how dysbiosis affects epidermal barrier function.
Moisturizers, including petrolatum, physiological lipid mixtures, and ceramide-dominant triple-physiolosic lipid (ceramide: cholesterol:free fatty acids at a 3:1:1 molar ratio), play critical roles in AD management.1101102 They improve clinical symptoms and skin barrier function, and reduces bacterial colonization.4102103104105106107 Petrolatum improves skin barrier functions by upregulation of AMPs, including LL-37, HBD-2, elafin, and S100 proteins.101 Additionally, epidermal differentiation markers, such as FLG and loricrin, are induced by moisturizers.4101 Moreover, petrolatum significantly reduces T-cell and dendritic cell infiltration in AD skin.101 Glatz et al.108 have reported that early emollient therapy alters the skin barrier and microbes in high-risk newborns. Of note, Nakatsuji et al.16 have demonstrated that application of coagulase-negative Staphylococcus strains to the skin of patients with AD decreases colonization by S. aureus.
It has been reported that use of dilute bleach (sodium hypochlorite) baths and intranasal mupirocin treatment improves AD symptoms.109 Other investigators have reported that topical use of bleach inhibites S. aureus and show beneficial effects on AD skin possibly through intrinsic anti-inflammatory effects.110111
Hyung et al.112 reported Lactobacillus strain, CJLP55, isolated from kimchi, reduced infiltration of mast cells, eosinophils, and production of Th2 cytokines in AD-induced mouse skin. Additionally, Notay et al.113 analyzed 315 articles and reported that probiotics and prebiotics improved AD symptoms including quality of life and clinical severity.
Recently, various types of immune therapy have been developed (Table 2). Clinical studies with broad and targeted therapies have been applied for patients with moderate-to-severe AD.1 Cyclosporine and oral glucocorticoids have been used, but there are limitations due to multiple adverse reactions. Dupilumab, anti-IL-4 Rα monoclonal antibody, improved clinical findings in adults with moderate-to-severe atopic dermatitis, without significant safety concerns.114115116117 Additionally, dupilumab up-regulated genes involved in skin barrier function.117 It will be interesting to learn if early treatment of AD with dupilumab could prevent progression of the atopic march.
Recent studies have demonstrated that moisturizers reduce rates of AD development4104105 and that probiotic supplementation may prevent AD.118119 Additionally, investigators have reported that skin commensal bacteria, including S. epidermidis and S. hominis, play crucial roles in skin homeostasis and defense against microbial penetration.474849 It has also been suggested that colonization by commensal staphylococci can modulate skin immunity and might prevent development of AD.1697 Therefore, correcting dysbiosis in AD skin may improve skin barrier function and prevent AD development. Recently, Kelleher et al.120 have demonstrated that increased TEWL at 2 days and 2 months predates and predicts AD at 1 year. Kim et al.121 have also reported that thymic stromal lymphopoietin (TSLP) predicts the development of AD during infancy. These data suggest that detection of increased TEWL, TSLP, and skin dysbiosis in early life might predict AD and facilitate introduction of strategies to prevent AD development. These would include early use of moisturizers, topical anti-inflammatory agents, probiotics as well as correction of microbial dysbiosis.
Factors, including immune dysregulation, epidermal gene mutations, deficiency of AMPs, and skin dysbiosis, may interact with each other and cause skin barrier defects. Several strategies have been utilized to improve skin barrier function and to control AD. Recently, moisturizers, probiotics, and targeted microbiome therapy have been suggested to prevent AD development in early life. Additionally, broad-spectrum and targeted therapies have been considered to control AD and prevent the atopic march in patients with moderate-to-severe AD. Further studies are warranted to determine the efficacy of these diverse strategies, including emollients, probiotics, and commensal bacteria, to prevent development of AD. It is noteworthy that recent data suggests AD is not just a local skin disease, but a systemic immune disease because nonlesional skin and blood profile show inflammatory findings. Therefore, we may need to expand our scope of management, in the future, to systemic treatment in patients with moderate-to-severe AD.
ACKNOWLEDGMENTS
The authors wish to acknowledge The Edelstein Family Foundation of Pediatric Allergy-Immunology for their generous support of this work. This work was also supported by USPHS grant R01 AR41256.
References
1. Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017; 139:S65–S76. PMID: 28390479.

2. Bieber T, D'Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schäppi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol. 2017; 139:S58–S64. PMID: 28390478.

3. Leung DY, Guttman-Yassky E. Assessing the current treatment of atopic dermatitis: unmet needs. J Allergy Clin Immunol. 2017; 139:S47–S48. PMID: 28390476.

4. Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017; 139:1723–1734. PMID: 28583445.

5. Ahn K. The prevalence of atopic dermatitis in Korean children. Allergy Asthma Immunol Res. 2016; 8:1–2. PMID: 26540495.

6. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006; 155:145–151. PMID: 16792766.

7. Rao DR, Sordillo JE, Kopel LS, Gaffin JM, Sheehan WJ, Hoffman E, et al. Association between allergic sensitization and exhaled nitric oxide in children in the School Inner-city Asthma Study. Ann Allergy Asthma Immunol. 2015; 114:256–257.e1. PMID: 25595887.

8. Boguniewicz M, Abramovits W, Paller A, Whitaker-Worth DL, Prendergast M, Cheng JW, et al. A multiple-domain framework of clinical, economic, and patient-reported outcomes for evaluating benefits of intervention in atopic dermatitis. J Drugs Dermatol. 2007; 6:416–423. PMID: 17668539.
9. Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008; 25:1–6.

10. Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014; 134:769–779. PMID: 25282559.

11. Mahdavinia M, Rasmussen HE, Engen P, Van den Berg JP, Davis E, Engen K, et al. Atopic dermatitis and food sensitization in South African toddlers: role of fiber and gut microbiota. Ann Allergy Asthma Immunol. 2017; 118:742–743.e3. PMID: 28583264.
12. Visitsunthorn N, Chatpornvorarux S, Pacharn P, Jirapongsananuruk O. Atopy patch test in children with atopic dermatitis. Ann Allergy Asthma Immunol. 2016; 117:668–673. PMID: 27979025.

13. Roerdink EM, Flokstra-de Blok BM, Blok JL, Schuttelaar ML, Niggemann B, Werfel T, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016; 116:334–338. PMID: 26947239.

14. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105. PMID: 25729616.

15. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011; 3:67–73. PMID: 21461244.

16. Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017; 9:eaah4680. PMID: 28228596.

17. Smith AR, Knaysi G, Wilson JM, Wisniewski JA. The skin as a route of allergen exposure: part I. Immune components and mechanisms. Curr Allergy Asthma Rep. 2017; 17:6. PMID: 28185161.

18. van Smeden J, Bouwstra JA. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016; 49:8–26. PMID: 26844894.

19. Busse D, Kudella P, Grüning NM, Gisselmann G, Ständer S, Luger T, et al. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J Invest Dermatol. 2014; 134:2823–2832. PMID: 24999593.

20. Erkoçoğlu M, Kocabaş CN. Role of IgA and IgM in severity of atopic dermatitis. Ann Allergy Asthma Immunol. 2015; 114:433.

21. Kim BE, Leung DY. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol Res. 2012; 4:12–16. PMID: 22211165.

22. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005; 6:328–340. PMID: 15803139.

23. Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001; 114:3069–3070. PMID: 11590230.

24. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008; 17:1063–1072. PMID: 19043850.

25. Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981; 69:271–318. PMID: 6163744.

26. Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002; 156:1099–1111. PMID: 11889141.
27. Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001; 31:279–294. PMID: 11251630.
28. Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992; 262:L647–L661. PMID: 1616050.

29. Jonca N, Guerrin M, Hadjiolova K, Caubet C, Gallinaro H, Simon M, et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem. 2002; 277:5024–5029. PMID: 11739386.

30. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009; 30:131–141. PMID: 19217824.

31. Nakatsuji T, Gallo RL. Antimicrobial peptides: old molecules with new ideas. J Invest Dermatol. 2012; 132:887–895. PMID: 22158560.

32. Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006; 24:341–348. PMID: 16546102.

33. Niyonsaba F, Nagaoka I, Ogawa H, Okumura K. Multifunctional antimicrobial proteins and peptides: natural activators of immune systems. Curr Pharm Des. 2009; 15:2393–2413. PMID: 19601839.

34. Kiatsurayanon C, Niyonsaba F, Smithrithee R, Akiyama T, Ushio H, Hara M, et al. Host defense (Antimicrobial) peptide, human β-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Invest Dermatol. 2014; 134:2163–2173. PMID: 24633129.

35. Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009; 11:220–228. PMID: 19115333.

36. De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011; 127:773–786.e1-7. PMID: 21163515.

37. Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005; 175:1776–1784. PMID: 16034119.
38. Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007; 127:594–604. PMID: 17068477.
39. Golec M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann Agric Environ Med. 2007; 14:1–4. PMID: 17655171.
40. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Allergy Asthma Rep. 2009; 9:265–272. PMID: 19656472.

41. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008; 121:1337–1343. PMID: 18329087.

42. Kezic S, Jakasa I. Filaggrin and skin barrier function. Curr Probl Dermatol. 2016; 49:1–7. PMID: 26844893.

43. Chng KR, Tay AS, Li C, Ng AH, Wang J, Suri BK, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol. 2016; 1:16106. PMID: 27562258.

44. Oh J, Conlan S, Polley EC, Segre JA, Kong HH. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012; 4:77. PMID: 23050952.

45. Oh J, Byrd AL, Deming C, Conlan S, Kong HH, NISC Comparative Sequencing Program, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014; 514:59–64. PMID: 25279917.

46. Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the human skin microbiome early in life. J Invest Dermatol. 2011; 131:2026–2032. PMID: 21697884.

47. Knaysi G, Smith AR, Wilson JM, Wisniewski JA. The skin as a route of allergen exposure: part II. Allergens and role of the microbiome and environmental exposures. Curr Allergy Asthma Rep. 2017; 17:7. PMID: 28210979.

48. Nakamizo S, Egawa G, Honda T, Nakajima S, Belkaid Y, Kabashima K. Commensal bacteria and cutaneous immunity. Semin Immunopathol. 2015; 37:73–80. PMID: 25326105.

49. Byrd AL, Deming C, Cassidy SK, Harrison OJ, Ng WI, Conlan S, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017; 9:eaal4651. PMID: 28679656.

50. Lee E, Lee SY, Kang MJ, Kim K, Won S, Kim BJ, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016; 117:91–92.e1. PMID: 27179583.

51. Tang KT, Ku KC, Chen DY, Lin CH, Tsuang BJ, Chen YH. Adult atopic dermatitis and exposure to air pollutants-a nationwide population-based study. Ann Allergy Asthma Immunol. 2017; 118:351–355. PMID: 28126434.

52. Knox SM, Erwin EA, Mosser-Goldfarb JL, Scherzer R. Sensitization patterns among patients with atopic dermatitis evaluated in a large tertiary care pediatric center. Ann Allergy Asthma Immunol. 2017; 118:645–647. PMID: 28372896.

53. Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998; 101:1614–1622. PMID: 9541491.

54. Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008; 128:1728–1736. PMID: 18200056.

55. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011; 365:1315–1327. PMID: 21991953.

56. Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010; 163:1333–1336. PMID: 21137118.
57. Kim BE, Bin L, Ye YM, Ramamoorthy P, Leung DY. IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with Th2 cytokines to enhance HSV-1 replication. J Invest Dermatol. 2013; 133:2678–2685. PMID: 23657503.

58. Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994; 94:870–876. PMID: 8040343.

59. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007; 120:150–155. PMID: 17512043.

60. Jang H, Matsuda A, Jung K, Karasawa K, Matsuda K, Oida K, et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J Invest Dermatol. 2016; 136:127–135. PMID: 26763432.

61. Nicotera P, Melino G. Caspase-14 and epidermis maturation. Nat Cell Biol. 2007; 9:621–622. PMID: 17541415.

62. Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007; 9:666–674. PMID: 17515931.

63. Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009; 124:485–493. 493.e1PMID: 19665780.

64. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008; 128:79–86. PMID: 17671515.

65. Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986; 115:84–92. PMID: 3516761.

66. Gutowska-Owsiak D, Schaupp AL, Salimi M, Taylor S, Ogg GS. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br J Dermatol. 2011; 165:492–498. PMID: 21564072.

67. Gutowska-Owsiak D, Schaupp AL, Salimi M, Selvakumar TA, McPherson T, Taylor S, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012; 21:104–110. PMID: 22229441.

68. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008; 126:332–337. PMID: 18166499.

69. Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009; 123:1361–1370.e7. PMID: 19501237.

70. Stemmler S, Parwez Q, Petrasch-Parwez E, Epplen JT, Hoffjan S. Two common loss-of-function mutations within the filaggrin gene predispose for early onset of atopic dermatitis. J Invest Dermatol. 2007; 127:722–724. PMID: 17008875.

71. Wan J, Mitra N, Hoffstad OJ, Margolis DJ. Influence of FLG mutations and TSLP polymorphisms on atopic dermatitis onset age. Ann Allergy Asthma Immunol. 2017; 118:737–738.e1. PMID: 28479194.

72. Yu HS, Kang MJ, Jung YH, Kim HY, Seo JH, Kim YJ, et al. Mutations in the filaggrin are predisposing factor in Korean children with atopic dermatitis. Allergy Asthma Immunol Res. 2013; 5:211–215. PMID: 23814674.

73. O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008; 122:689–693. PMID: 18774165.
74. Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008; 121:872–877.e9. PMID: 18325573.

75. Gruber R, Börnchen C, Rose K, Daubmann A, Volksdorf T, Wladykowski E, et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am J Pathol. 2015; 185:2777–2789. PMID: 26319240.

76. Leclerc EA, Huchenq A, Mattiuzzo NR, Metzger D, Chambon P, Ghyselinck NB, et al. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J Cell Sci. 2009; 122:2699–2709. PMID: 19596793.

77. Lee UH, Kim BE, Kim DJ, Cho YG, Ye YM, Leung DY. Atopic dermatitis is associated with reduced corneodesmosin expression: role of cytokine modulation and effects on viral penetration. Br J Dermatol. 2017; 176:537–540.

78. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003; 171:3262–3269. PMID: 12960356.

79. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002; 347:1151–1160. PMID: 12374875.

80. Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010; 163:659–661. PMID: 20545685.

81. Brauweiler AM, Goleva E, Leung DY. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol. 2014; 134:2114–2121. PMID: 24468745.

82. Son ED, Kim HJ, Park T, Shin K, Bae IH, Lim KM, et al. Staphylococcus aureus inhibits terminal differentiation of normal human keratinocytes by stimulating interleukin-6 secretion. J Dermatol Sci. 2014; 74:64–71. PMID: 24398033.

83. Brauweiler AM, Hall CF, Goleva E, Leung DY. Staphylococcus aureus lipoteichoic acid inhibits keratinocyte differentiation through a p63-mediated pathway. J Invest Dermatol. 2017; 137:2030–2033. PMID: 28528912.

84. Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006; 117:836–841. PMID: 16630942.

85. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013; 62:151–161. PMID: 23712284.

86. Danso M, Boiten W, van Drongelen V, Gmelig Meijling K, Gooris G, El Ghalbzouri A, et al. Altered expression of epidermal lipid biosynthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J Dermatol Sci. 2017; 88:57–66. PMID: 28571749.

87. Kim D, Lee NR, Park SY, Jun M, Lee K, Kim S, et al. As in atopic dermatitis, nonlesional skin in allergic contact dermatitis displays abnormalities in barrier function and ceramide content. J Invest Dermatol. 2017; 137:748–750. PMID: 27826010.
88. Ito S, Ishikawa J, Naoe A, Yoshida H, Hachiya A, Fujimura T, et al. Ceramide synthase 4 is highly expressed in involved skin of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2017; 31:135–141. PMID: 27358008.

89. Li S, Villarreal M, Stewart S, Choi J, Ganguli-Indra G, Babineau DC, et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br J Dermatol. 2017; 177:e125–e127. PMID: 28244066.

90. Oh MJ, Nam JJ, Lee EO, Kim JW, Park CS. A synthetic C16 omegahydroxyphytoceramide improves skin barrier functions from diversely perturbed epidermal conditions. Arch Dermatol Res. 2016; 308:563–574. PMID: 27402316.

91. Lowe AJ, Su JC, Allen KJ, Abramson MJ, Cranswick N, Robertson CF, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. Br J Dermatol. 2017; Forthcoming.
92. Eyerich K, Eyerich S, Biedermann T. The multi-modal immune pathogenesis of atopic eczema. Trends Immunol. 2015; 36:788–801. PMID: 26602548.

93. Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015; 42:756–766. PMID: 25902485.

94. Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012; 337:1115–1119. PMID: 22837383.

95. Zeeuwen PL, Boekhorst J, van den Bogaard EH, de Koning HD, van de Kerkhof PM, Saulnier DM, et al. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012; 13:R101. PMID: 23153041.

97. Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WH, Murray D, et al. Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017; 139:166–172. PMID: 27609659.
98. Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013; 93:261–267. PMID: 23322028.

99. Rippke F, Schreiner V, Schwanitz HJ. The acidic milieu of the horny layer: new findings on the physiology and pathophysiology of skin pH. Am J Clin Dermatol. 2002; 3:261–272. PMID: 12010071.
100. Brauweiler AM, Goleva E, Leung DY. Interferon-γ protects from staphylococcal alpha toxin-induced keratinocyte death through apolipoprotein L1. J Invest Dermatol. 2016; 136:658–664. PMID: 27015454.

101. Czarnowicki T, Malajian D, Khattri S, Correa da Rosa J, Dutt R, Finney R, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016; 137:1091–1102.e7. PMID: 26431582.

102. Lee HJ, Lee SH. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol Res. 2014; 6:276–287. PMID: 24991450.

103. Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017; 139:S49–S57. PMID: 28390477.
104. Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol. 2010; 63:587–593. PMID: 20692725.

105. Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014; 134:818–823. PMID: 25282563.

106. Cardona ID, Stillman L, Jain N. Does bathing frequency matter in pediatric atopic dermatitis? Ann Allergy Asthma Immunol. 2016; 117:9–13. PMID: 27371966.

107. Pabst RC, Starr KP, Qaiyumi S, Schwalbe RS, Gewolb IH. The effect of application of aquaphor on skin condition, fluid requirements, and bacterial colonization in very low birth weight infants. J Perinatol. 1999; 19:278–283. PMID: 10685239.

108. Glatz M, Polley E, Simpson E, Kong H. Emollient therapy alters skin barrier and microbes in infants at risk for developing atopic dermatitis. J Invest Dermatol. 2015; 135:S31.
109. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009; 123:e808–e814. PMID: 19403473.

110. Eriksson S, van der Plas MJ, Mörgelin M, Sonesson A. Antibacterial and antibiofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br J Dermatol. 2017; 177:513–521. PMID: 28238217.
111. Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight. 2016; 1:e86955. PMID: 27478874.

112. Hyung KE, Kim SJ, Jang YW, Lee DK, Hyun KH, Moon BS, et al. Therapeutic effects of orally administered CJLP55 for atopic dermatitis via the regulation of immune response. Korean J Physiol Pharmacol. 2017; 21:335–343. PMID: 28461776.

113. Notay M, Foolad N, Vaughn AR, Sivamani RK. Probiotics, prebiotics, and synbiotics for the treatment and prevention of adult dermatological diseases. Am J Clin Dermatol. 2017; 18:721–732. PMID: 28681230.

114. Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016; 375:2335–2348. PMID: 27690741.

115. Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016; 387:40–52. PMID: 26454361.

116. Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014; 371:130–139. PMID: 25006719.

117. Hamilton JD, Suárez-Fariñas M, Dhingra N, Cardinale I, Li X, Kostic A, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014; 134:1293–1300. PMID: 25482871.

118. Rø AD, Simpson MR, Rø TB, Storrø O, Johnsen R, Videm V, et al. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy. 2017; 47:1014–1021. PMID: 28346719.

119. di Mauro G, Bernardini R, Barberi S, Capuano A, Correra A, De'Angelis GL, et al. Prevention of food and airway allergy: consensus of the Italian Society of Preventive and Social Paediatrics, the Italian Society of Paediatric Allergy and Immunology, and Italian Society of Pediatrics. World Allergy Organ J. 2016; 9:28. PMID: 27583103.

120. Kelleher M, Dunn-Galvin A, Hourihane JO, Murray D, Campbell LE, McLean WH, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015; 135:930–935.e1. PMID: 25618747.
121. Kim J, Kim BE, Lee J, Han Y, Jun HY, Kim H, et al. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J Allergy Clin Immunol. 2016; 137:1282–1285.e4. PMID: 26879860.

Fig. 1
Impaired skin barrier enhances allergen penetration and activates the innate immune system. Multiple factors, including immune dysregulation, defects in terminal epithelial differentiation such as lack of filaggrin (FLG), deficiency of antimicrobial peptides (AMPs), altered composition of stratum corneum intercellular lipids, and altered skin microbiome cause skin barrier defects. Source: Czarnowicki et al. J Allergy Clin Immunol 2017;139:1723–34.
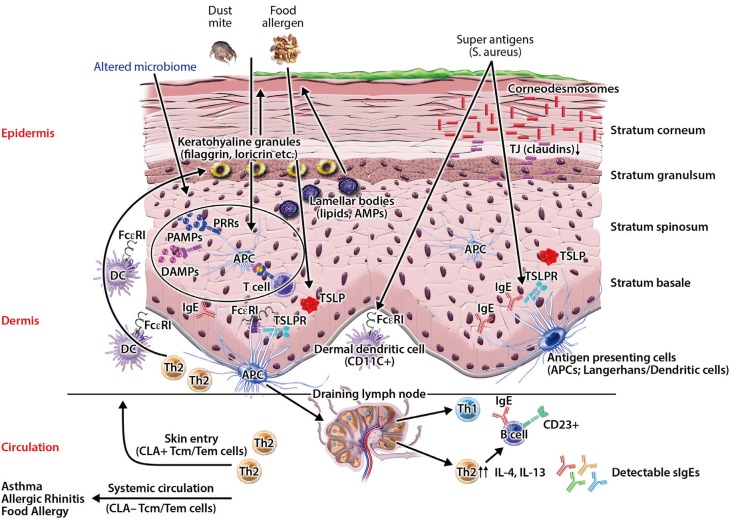
Fig. 2
Keratinocytes differentiated in the presence of IL-4 and IL-13 exhibit significantly reduced filaggrin. Primary human keratinocytes were cultured for 5 days in 0.06 or 1.3 mmol/L CaCl2 in the presence of IL-4 plus IL-13 or interferon (IFN)-gamma. *P<0.05; ***P<0.001 between the exposure groups. Source: Howell et al. J Allergy Clin Immunol 2007;120:150–5.
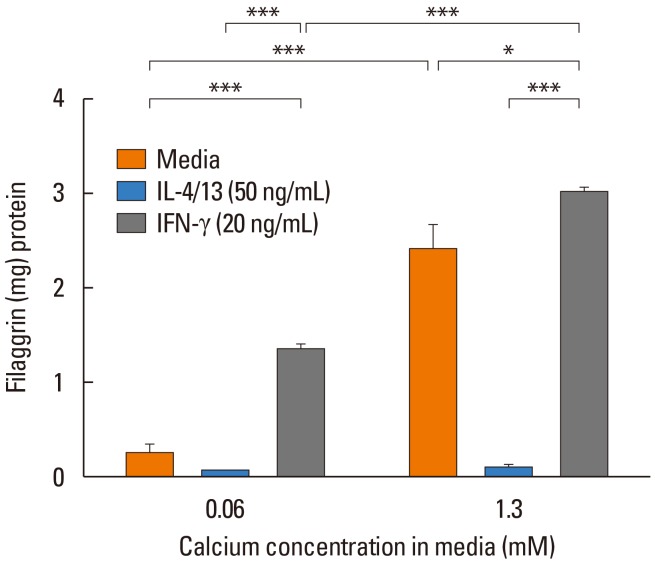
Table 1
Epidermal Barrier Dysfunction in atopic dermaitis
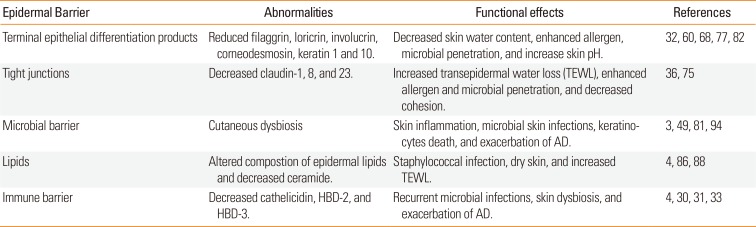
| Epidermal Barrier | Abnormalities | Functional effects | References |
|---|---|---|---|
| Terminal epithelial differentiation products | Reduced filaggrin, loricrin, involucrin, corneodesmosin, keratin 1 and 10. | Decreased skin water content, enhanced allergen, microbial penetration, and increase skin pH. | 3260687782 |
| Tight junctions | Decreased claudin-1, 8, and 23. | Increased transepidermal water loss (TEWL), enhanced allergen and microbial penetration, and decreased cohesion. | 3675 |
| Microbial barrier | Cutaneous dysbiosis | Skin inflammation, microbial skin infections, keratinocytes death, and exacerbation of AD. | 3498194 |
| Lipids | Altered compostion of epidermal lipids and decreased ceramide. | Staphylococcal infection, dry skin, and increased TEWL. | 48688 |
| Immune barrier | Decreased cathelicidin, HBD-2, and HBD-3. | Recurrent microbial infections, skin dysbiosis, and exacerbation of AD. | 4303133 |
Table 2
Recent controlled trails in patients with atopic dermatitis
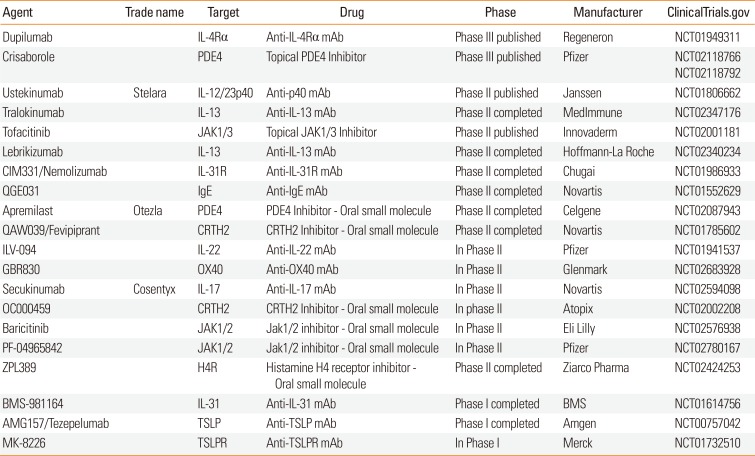
| Agent | Trade name | Target | Drug | Phase | Manufacturer | www.ClinicalTrials.gov |
|---|---|---|---|---|---|---|
| Dupilumab | IL-4Rα | Anti-IL-4Rα mAb | Phase III published | Regeneron | NCT01949311 | |
| Crisaborole | PDE4 | Topical PDE4 Inhibitor | Phase III published | Pfizer |
NCT02118766 NCT02118792 |
|
| Ustekinumab | Stelara | IL-12/23p40 | Anti-p40 mAb | Phase II published | Janssen | NCT01806662 |
| Tralokinumab | IL-13 | Anti-IL-13 mAb | Phase II completed | MedImmune | NCT02347176 | |
| Tofacitinib | JAK1/3 | Topical JAK1/3 Inhibitor | Phase II published | Innovaderm | NCT02001181 | |
| Lebrikizumab | IL-13 | Anti-IL-13 mAb | Phase II completed | Hoffmann-La Roche | NCT02340234 | |
| CIM331/Nemolizumab | IL-31R | Anti-IL-31R mAb | Phase II completed | Chugai | NCT01986933 | |
| QGE031 | IgE | Anti-IgE mAb | Phase II completed | Novartis | NCT01552629 | |
| Apremilast | Otezla | PDE4 | PDE4 Inhibitor - Oral small molecule | Phase II completed | Celgene | NCT02087943 |
| QAW039/Fevipiprant | CRTH2 | CRTH2 Inhibitor - Oral small molecule | Phase II completed | Novartis | NCT01785602 | |
| ILV-094 | IL-22 | Anti-IL-22 mAb | In Phase II | Pfizer | NCT01941537 | |
| GBR830 | OX40 | Anti-OX40 mAb | In Phase II | Glenmark | NCT02683928 | |
| Secukinumab | Cosentyx | IL-17 | Anti-IL-17 mAb | In Phase II | Novartis | NCT02594098 |
| OC000459 | CRTH2 | CRTH2 Inhibitor - Oral small molecule | In phase II | Atopix | NCT02002208 | |
| Baricitinib | JAK1/2 | Jak1/2 inhibitor - Oral small molecule | In Phase II | Eli Lilly | NCT02576938 | |
| PF-04965842 | JAK1/2 | Jak1/2 inhibitor - Oral small molecule | In Phase II | Pfizer | NCT02780167 | |
| ZPL389 | H4R | Histamine H4 receptor inhibitor - Oral small molecule | Phase II completed | Ziarco Pharma | NCT02424253 | |
| BMS-981164 | IL-31 | Anti-IL-31 mAb | Phase I completed | BMS | NCT01614756 | |
| AMG157/Tezepelumab | TSLP | Anti-TSLP mAb | Phase I completed | Amgen | NCT00757042 | |
| MK-8226 | TSLPR | Anti-TSLPR mAb | In Phase I | Merck | NCT01732510 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download