Abstract
Palonosetron is a 5-hydroxytryptamine-3 (5-HT-3) receptor antagonist used for preventing postoperative nausea and vomiting. Compared with ondansetron and granisetron, it is a better drug because of prolonged action and minimal side effects. Some adverse effects of palonosetron have been reported. In this report, we describe a 37-year-old male who developed severe hypersensitivity reactions to palonosetron during surgery for kidney donation. His medical history was unremarkable, except for inguinal hernia with herniorrhaphy 8 years ago. The surgery was uneventful until 2 hours 20 minutes. After palonosetron injection, his blood pressure dropped to 80/50 mm Hg, and facial edema, rash, conjunctival swelling, and wheezing developed. The patient was resuscitated by administration of ephedrine, hydrocortisone, and peniramine. Following the surgery, the patient was monitored for 3 days, and there were no subsequent anaphylactic reactions or other complications. The skin test on postoperative day 54 was positive for hypersensitivity to palonosetron. Although palonosetron is known for its safety, other hypersensitivity events have been reported. Ondansetron is another widely used 5-HT-3 antagonist, which has been reported to cause anaphylaxis. Therefore, clinicians should be aware of the possibility of patients experiencing severe adverse reactions to palonosetron.
Anaphylactic response to drugs manifests as a respiratory distress due to laryngeal edema and intense bronchospasm, often followed by vascular collapse or shock.1 Several redisposing factors are associated with hypersensitivity reactions to drugs in patients. Antibiotics, anesthetics agents, non-steroidal anti-inflammatory drugs, and opiates are common triggers of anaphylaxis.2
Severe hypersensitivity reaction during general anesthesia is one of the most frequent drug-induced anaphylactic reactions, as recently shown by several studies in the United States.3 Diagnosis of perioperative anaphylaxis is complicated as the patient is often sedated and thus unable to alert the physician of symptoms. Additionally, diagnosis can be hampered by obstruction of skin manifestations with surgical drapes, and cardiac events associated with anaphylaxis can be mistaken for other causes of cardiovascular collapse. Furthermore, multiple drugs are frequently administered simultaneously or in rapid succession during surgery. Most hypersensitivity reactions occur within minutes after administration of the drug; however, depending on the agent and time of administration, reactions can be late-onset.4 Common drugs causing anaphylaxis include neuromuscular blocking agents, latex, antibiotics, hypnotics, opioids, and colloids.
Palonosetron is a 5-hydroxytryptamine3 (5-HT3) antagonist used for the prevention and treatment of nausea and vomiting due to cancer chemotherapy and surgery. Other commonly used 5-HT3 antagonists include ondansetron and granisetron (Fig. 1). Additionally, ondansetron- and granisetron-related hypersensitivity reactions have been reported in 8 and 2 patients, respectively.5,6,7,8,9,10
In this report, we describe a patient who developed anaphylaxis during general anesthesia following intravenous palonosetron administration.
A 37-year-old male was admitted for kidney donation. Prior to surgery, he underwent the following tests for routine evaluation: dimercaptosuccinic acid (DMSA) renal scan, pulmonary function test, abdominopelvic computed tomography, and echocardiogram. His left atrium was mildly enlarged (42 mm), as seen from the echocardiogram; however, no other abnormalities were found. Serological tests showed that the patient was negative for cytomegalovirus, human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.
As shown in Fig. 2, for general anesthesia, rocuronium, fentanyl, and sodium pentothal were administered for intubation, and desflurane was used for maintenance. The surgery was uneventful until administration of palonosetron at 2 hours 20 minutes after the start of the surgery. Five minutes later, the patient's blood pressure (BP) decreased from 140/90 at baseline to 80/50 mm Hg, his heart rate increased from 65 to 80 per minutes, and his oxygen saturation was 98%. During resuscitation, 5 mg of ephedrine was administered twice, but no response was achieved; thus, 10 mg of ephedrine was administered 2 more times. Thirty minutes after palonosetron administration, the patient developed facial and lip edema, conjunctival swelling, and rashes on the face and chest. Thus, 100 mg of hydrocortisone and 4 mg of chlorpheniramine were intravenously administered for treating the rash. Physical examination performed at that time revealed generalized wheezing over both lungs. Therefore, a salbutamol nebulizer (Ventolin) was administered. The results of laboratory tests conducted for differential diagnosis were as follows: blood glucose, 156 mg/dL; sodium, 137 mmol/L; potassium, 3.6 mmol/L; chloride, 105 mmol/L; lactate, 2.2 mmol/L; and hemoglobin, 13.6 g/dL. Thus, bleeding and hypovolemia were excluded from the differential diagnosis. No other intraoperative complications were found.
After the surgery, the patient was admitted to the intensive care unit for close monitoring, and delayed anaphylaxis was not observed. No further reaction was reported over the following 72 hours. Serum tryptase levels measured 3 hours and 1 day after anaphylaxis were 8.60 and 1.20 µg/L, respectively.
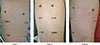
The patient had no prior history of allergies to drugs/food or history of allergic dermatitis, rhinitis, or asthma. He had undergone herniorrhaphy eight years ago, with no adverse events. However, given the severity of the hypersensitivity reaction, a skin test was performed to evaluate the patient's response to palonosetron on days 1, 17, and 54 following the surgery. The skin test was negative on days 1 and 17, but was positive at 1:100 dilution of the allergen (0.5 µg/mL) on day 54 (Fig. 3).
Palonosetron is a second-generation 5-HT3 receptor antagonist used for postoperative nausea and vomiting (PONV). Palonosetron is superior to ondansetron and granisetron for PONV prophylaxis due to prolonged duration and minimal side effects.11 The most common adverse effects of palonosetron in PONV are QT prolongation, bradycardia, headache, and constipation.12
This is the first report of a patient who developed anaphylaxis to palonosetron in Korea. While 1 or more of the anesthetics used in the perioperative period, namely rocuronium, sodium pentothal, and fentanyl, could potentially cause anaphylaxis, they were unlikely to cause serious conditions. Anaphylaxis is an immediate reaction, and patient's symptoms, starting with hypotension, emerged 2 hours after anesthesia induction, within a few minutes after palonosetron injection. The incidence of anaphylaxis to palonosetron is low; however, there was a strong correlation between the time after palonosetron administration and the symptoms. Thus, the patient was diagnosed with anaphylactic reactions to palonosetron.
Review of the literature revealed 2 previous case reports of anaphylactic reactions to palonosetron. One case involved a 40-year-old female who developed hypotension and hypoxia within minutes following palonosetron administration.7 The second case was of a 40-year-old female patient who developed hypotension and bradycardia, and was resuscitated with fluid and diphenhydramine.9 No other cases with definitive anaphylaxis diagnosis were reported.
Palonosetron is a relatively new drug with unexpected side effects reported in a post-marketing survey.12 The other relatively well-known 5-HT3 antagonist, ondansetron, was also reported to have an association with hypersensitivity.6,7,8,9,10 Two patients who developed anaphylaxis following ondansetron administration were assessed by using an intradermal test, which was positive at ondansetron doses of 0.02 and 0.002 mg/mL, respectively. Intriguingly, the skin prick test was negative in both patients.
In recent reports, a patient who experienced ondansetron-related hypersensitivity had no reaction to granisetron or palonosetron.5,8 Ondansetron, granisetron, and palonosetron do not share a common indole ring, which might be one of the reasons for the lack of cross-reactivity between these 5-HT3 antagonists.
In summary, as illustrated in this case of intraoperative anaphylaxis, albeit rare, palonosetron should be considered as a cause of anaphylaxis.
Figures and Tables
Fig. 2
Chart summarizing the vital signs of the patient with anaphylaxis. Arrows indicate drug administrations: the first narrow arrow, 450 mg of pentothal sodium, 60 mg of rocuronium, and 50 µg of fentanyl; the second narrow arrow, 50 µg of fentanyl; arrowhead, palonosetron; and wide arrow, 5 mg of ephedrine twice, followed by 10 mg of ephedrine twice. Circle indicates the appearance of rash, facial edema, and conjunctival swelling with immediate administration of 4 mg of chlorpheniramine and 100 mg of hydrocortisone. sBP, systolic blood pressure; dBP, diastolic blood pressure.

Fig. 3
Skin test to determine hypersensitivity to palonosetron at dilutions of 1:1, 1:10, 1:100, and 1:1,000. H, control (histamine); N, control (saline). On POD 54, the sizes of wheals were as follows: histamine, 9.5 mm; saline, 2 mm; palonosetron 1:1, 13.86 mm; palonosetron 1:10, 8.5 mm; palonosetron 1:100, 9.5 mm; and palonosetron, 1:1,000, no wheal.
POD, postoperative day.
References
1. Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions: S2K-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Dermatological Society (DDG) in collaboration with the Association of German Allergologists (AeDA), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Swiss Society for Allergy and Immunology (SGAI), the Austrian Society for Allergology and Immunology (ÖGAI), the German Academy of Allergology and Environmental Medicine (DAAU), the German Center for Documentation of Severe Skin Reactions and the German Federal Institute for Drugs and Medical Products (BfArM). Allergo J Int. 2015; 24:94–105.
2. Laemmle-Ruff I, O’Hehir R, Ackland M, Tang ML. Anaphylaxis -identification, management and prevention. Aust Fam Physician. 2013; 42:38–42.
3. Mirone C, Preziosi D, Mascheri A, Micarelli G, Farioli L, Balossi LG, et al. Identification of risk factors of severe hypersensitivity reactions in general anaesthesia. Clin Mol Allergy. 2015; 13:11.
4. Kannan JA, Bernstein JA. Perioperative anaphylaxis: diagnosis, evaluation, and management. Immunol Allergy Clin North Am. 2015; 35:321–334.
5. Demir HA, Batu ED, Yalçın B, Civelek E, Saçkesen C, Büyükpamukçu M. Anaphylactic reaction owing to ondansetron administration in a child with neuroblastoma and safe use of granisetron: a case report. J Pediatr Hematol Oncol. 2010; 32:e341–e342.
6. Fernando SL, Broadfoot AJ. Ondansetron anaphylaxis: a case report and protocol for skin testing. Br J Anaesth. 2009; 102:285–286.
7. Gupta YK, Shanmugam SP, Padhy BM, Goyal A. Palonosetron induced anaphylaxis in an adult female. Br J Clin Pharmacol. 2010; 70:149–150.
8. Leung J, Guyer A, Banerji A. IgE-mediated hypersensitivity to ondansetron and safe use of palonosetron. J Allergy Clin Immunol Pract. 2013; 1:526–527.
9. Pietkiewicz JM. Possible anaphylaxis to palonoestron. J Oncol Pharm Pract. 2012; 18:296–298.
10. Tan J, Mehr S. Anaphylaxis to an ondansetron wafer. J Paediatr Child Health. 2012; 48:543–544.
11. Gupta K, Singh I, Gupta PK, Chauhan H, Jain M, Rastogi B. Palonosetron, Ondansetron, and Granisetron for antiemetic prophylaxis of postoperative nausea and vomiting - a comparative evaluation. Anesth Essays Res. 2014; 8:197–201.
12. Helsinn Healthcare SA (CH). Palonosetron prescribing information [Internet]. Woodcliff Lake (NJ): Eisai Inc.;2014. cited 2015 Oct 27. Available from: http://www.aloxi.com.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


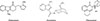
 XML Download
XML Download