Abstract
Purpose
Recent evidence suggests a global burden of chronic cough in general populations. However, the definitions vary greatly among epidemiological studies, and none have been validated for clinical relevance. We aimed to examine previous epidemiological definitions in detail and explore the operational characteristics.
Methods
A systematic review was conducted for epidemiological surveys that reported the prevalence of chronic cough in general adult populations during the years 1980 to 2013. A literature search was performed on Pubmed and Embase without language restriction. Epidemiological definitions for chronic cough were classified according to their components, such as cutoff duration. Meta-analyses were performed for the male-to-female ratio of chronic cough prevalence to explore operational characteristics of epidemiological definitions.
Results
A total of 70 studies were included in the systematic review. The most common epidemiological definition was identified as 'cough ≥3 months' duration without specification of phlegm (n=50); however, it conflicted with the cutoff duration in current clinical guidelines (cough ≥8 weeks). Meta-analyses were performed for the male-to-female ratio of chronic cough among 28 studies that reported sex-specific prevalence using the most common definition. The pooled male-to-female odds ratio was 1.26 (95% confidence interval 0.92-1.73) with significant heterogeneity (I2=96%, P<0.001), which was in contrast to clinical observations of female predominance from specialist clinics. Subgroup analyses did not reverse the ratio or reduce the heterogeneity.
Conclusions
This study identified major issues in defining chronic cough in future epidemiological studies. The conflict between epidemiological and clinical diagnostic criteria needs to be resolved. The unexpected difference in the gender predominance between the community and clinics warrants further studies. Clinical validation of the existing definition is required.
Cough is an essential mechanism for protecting airways, but is also one of the most common symptoms that lead patients to seek medical attention.1 In particular, chronic cough has a high global health burden, affecting about 10% of general adult populations.2 Chronic cough is a significant health issue due to its substantial impact on quality of life,3 and it causes many clinical challenges.45
In the past, chronic cough was understood to be a consequence of several diseases affecting airway sensory nerve terminals, such as rhinitis, gastroesophageal acid reflux, and eosinophilic airway diseases.45 However, many patients with these diseases do not report cough,67 suggesting that chronic cough, although associated, may be a separate condition. Moreover, diagnostic and therapeutic failures have been reported in a substantial proportion of patients with the classic causes of chronic cough (12%-44%).8 Therefore, cough hypersensitivity syndrome, a new paradigm has recently been formulated to understand chronic cough as a clinical syndrome with a common intrinsic pathophysiology (cough hypersensitivity).9101112
This recent paradigm shift in understanding chronic cough warrants further epidemiological characterization. However, in our recent meta-analyses of prevalence studies, substantial methodological heterogeneity was found among the existing epidemiological definitions.2 Moreover, to the best of our knowledge, none of these definitions have been validated for clinical relevance. To further advance understanding of this disease, a consensus definition needs to be developed. Using a systematic literature review, this study examined previous epidemiological definitions utilized in general population surveys reporting the prevalence of chronic cough and explored the operational characteristics of the most common definition. We hope to open a field of discussion toward consensus development for the definition of chronic cough in further epidemiological studies.
A systematic search was conducted of the Pubmed and Embase databases for the literature that measured the prevalence of chronic cough in community-based adult populations, as previously described.2 The search terms were "cough AND (epidemiology OR epidemiologic OR epidemiological OR prevalence OR incidence)" for articles published in peer-reviewed journals between January 1980 and December 2013. Additional searches were performed in Google Scholar (http://scholar.google.com) and via cross-referenced articles. Language was not restricted. In cases where full-text links were not available, we contacted the corresponding authors by email for the full text. The systematic review process followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Fig. 1).13
Studies were initially included if they met the following criteria: (1) cross-sectional or longitudinal studies conducted in community-based or unselected adult populations and (2) reported prevalence of chronic cough. Various definitions of chronic cough were accepted due to the lack of a validated definition. However, here we ultimately included studies only if their diagnostic terms were chronic cough, or other conceptually equivalent terms, such as long-term, longstanding, or persistent cough. We excluded studies with bronchitis, or cough but without including any conceptual terms for chronicity in their diagnostic labels. Other exclusion criteria were as follows: (1) no relevant analyses, (2) convenience sample studies without detailed information on demographic characteristics, (3) birth cohort studies confined to specific ages, (4) studies confined to specific occupations, (5) studies using duplicate samples, or (6) nonoriginal papers (reviews and case reports). In determining eligibility, discrepancies were resolved through consensus conference.
For all of the included articles, we extracted and confirmed the data. The extracted outcomes included study design, study region, study year, participant characteristics, smoking rate, and definition and prevalence of chronic cough. Sex-specific prevalence of chronic cough was also extracted in as many cases as possible, including email contact of the corresponding authors. In addition, according to text excerpts of research purposes described in original papers, individual studies were classified as studies with chronic cough as the primary outcome or not.
Quality was assessed by 2 reviewers using a quality scoring system in a quality effects model.14 Briefly, the scores were calculated by summing 6 measurements: (1) definition of the study population, (2) use of reported diagnostic criteria, (3) method of case ascertainment, (4) administration of measurement protocol, (5) characteristics of catchment area, and (6) prevalence measure. Discrepancies in scoring were resolved by consensus conference.
Definitions were classified according to the diagnostic terms and questions as utilized in original papers. Definitions were also examined by their components, such as cutoff duration, phlegm, or prevalence measure. Time trends in the definitions were also examined by each decade (1980-1990, 1991-2000, and 2001-2013). These evaluations were repeated for a subgroup of studies that had chronic cough as the primary outcome.
Operational characteristics of the most common definition were examined in relation to sex ratio, as female predominance was a representative finding among patients visiting cough clinics in several countries.15 A quality effects model was primarily used to calculate a pooled male-to-female odds ratio (OR) and 95% confidence interval (95% CI) of chronic cough prevalence. Heterogeneity was assessed using the I2 test, with a cutoff of 50%16 and the chi-squared test with a P value <0.10. We adapted the quality effects model due to a considerable heterogeneity in our previous meta-analyses of prevalence,2 probably due to a combination of true variance of the prevalence and variability produced by the methodological differences used to measure the outcome.14 The quality effects model gives greater weight to studies of high quality than those of low quality, as it uses the quality scores assigned to individual studies; however, the random effects model assigns more weight to smaller studies in cases of heterogeneity.17 Therefore, we determined that the quality effects model was a more appropriate statistical method to obtain a pooled ratio. However, we also presented the pooled estimates by the random effects model for comparison.
Subgroup analyses were performed to explain the heterogeneity, according to study year (before vs after 2000), region, age group (non-elderly vs elderly), and study quality (quality score ≤9 vs ≥10). Evidence of publication bias was assessed by applying the Peters test to funnel plots of the natural log of OR.18 Quality effects meta-analyses were performed using MetaXL software version 2.0 (http://www.epigear.com), and all other statistical analyses were conducted using Stata software package release 12.0 (Stata Corp., College Station, TX, USA).
A total of 70 articles met the inclusion criteria19202122232425262728293031323334353637383940414243444546474849505152535455565758596061626364656667686970717273747576777879808182838485868788 (Fig. 1). Nineteen articles8990919293949596979899100101102103104105106107 that had been included in previous metaanalyses for prevalence2 were excluded here because their diagnostic terms were determined not to specifically intend chronic cough (such as 'chronic bronchitis', 'frequent cough', or just 'cough' in their terms). The characteristics of the 70 articles included and the 19 article excluded are summarized in Appendix Tables S1 and S2, respectively. The quality scores of the included studies are described in Appendix Table S3.
Several diagnostic terms were identified from the 70 studies included, such as chronic cough (n=56), chronic cough with phlegm (n=4), long-standing cough (n=5), long-term cough (n=2), and persistent cough (n=3) (Appendix Table S4). By cutoff duration, cough ≥3 months was by far the most common criterion (n=55), and cough ≥8 weeks was the second (n=3) most common. Eleven studies did not specify a cutoff duration for defining chronic cough (Table 1).
Based on the research purpose of individual articles (Appendix Table S3), 13 studies were classified as having chronic cough as the primary outcome. Cough ≥3 months was again the most common cutoff criterion (n=7), and cough ≥8 weeks was the second (n=3) most common. One study used cough ≥3 weeks, and the remaining 2 studies did not use a cutoff duration for cough (Table 1).
Time trends in the utilization of cutoff duration for defining chronic cough were examined (Fig. 2). Over the last 3 decades, the overall number of epidemiological studies for chronic cough had continuously increased, but cough ≥3 months remained the most frequently used criterion. Other cutoff criteria, such as 8 or 3 weeks, have only been found in recent studies published during 2001-2013. In a subgroup analysis of 13 studies with chronic cough as the primary outcome, cough ≥3 months remained the most common criterion, but the proportion of cough ≥8 weeks was higher than in the analysis of all studies (Fig. 2).
Most studies did not differentiate by the presence of phlegm in their reporting the prevalence of chronic cough (n=64). This preponderance was similarly observed in a subgroup analysis of 13 studies with chronic cough as the primary outcome (Table 1). Of 6 studies that utilized a phlegm component, none quantified or specified the diagnostic criteria for phlegm in detail. Based on the ways of measuring prevalence, the majority of studies (n=60) were classified as using period prevalence (12-month prevalence), whereas 6 studies used point prevalence (Table 1). The proportion of using point prevalence was higher in the subgroup of studies that analyzed chronic cough as the primary outcome (5 of 13).
Next, we examined the operational characteristics of the most common definition (n=50, cough ≥3 months [12-month prevalence] with no specification on phlegm, i.e., using a question, "Do you usually cough on most days for 3 consecutive months or more during the year?") in relation to a male-to-female ratio of chronic cough prevalence because female predominance is a common finding among patients visiting cough clinics in several countries.15 This meta-analysis included 28 studies (56%) that specified sex-specific prevalence.19202327283233363742444546525355606467687072737677798188 Sex-specific pooled prevalence was 9.6% (95% CI 6.0-14.1%, I2=99%, P<0.001) in males, and 8.6% (95% CI 5.2-12.8%, I2=99%, P<0.001) in females by the quality effects model. The pooled male-to-female ratio of chronic cough prevalence was OR 1.26 (95% CI 0.92-1.73) (Fig. 3) with a significant level of heterogeneity (I2=96%, P<0.001) but no significant funnel plot asymmetry (Peters test, P=0.592). Male preponderance was also observed by random effects model analyses (OR 1.45, 95% CI 1.20-1.75; Appendix Fig. S1).
The current smoking rate was correlated with chronic cough prevalence at population levels (r=0.378, P=0.009), and was markedly higher among males than females (40.8±13.3% vs 13.7±17.7%, P<0.001). Thus, smoking was presumed to be a confounder in the association between gender and chronic cough prevalence. However, meta-regression analyses for sex-smoking-cough interactions were not performed here, as only 15 studies reported a sex-specific rate of current smoking.
We performed subgroup analyses as follows, to explore confounders affecting the gender/cough association. In subgroup analyses by study year, the male-to-female ratio was slightly lower in recent studies conducted after 2000, compared to studies before 2000 (Table 2); however, significant heterogeneity still remained among recent studies after 2000 (I2=76%, P<0.001). In subgroup analyses by age group, elderly-specific studies337376 had a lower heterogeneity than non-elderly studies20233642444552556467707279; however, the number of elderly-specific studies was small (n=3). Subgroup analyses by study quality score or region also did not significantly reduce heterogeneity (Table 2).
In previous analyses, we found a global pooled prevalence of chronic cough of 9.6% with a wide geographical distribution.2 Here, we examined previous epidemiological definitions and their differences, and explored the operational characteristics of the most common definition in relation to the male-to-female ratio.
The high frequency of using 3 months as the cutoff for chronic cough in epidemiological studies may be attributed to the use of major standardized questionnaires in respiratory epidemiology. The British Medical Research Council (BMRC) questionnaire was the first one, particularly developed for the epidemiology of chronic bronchitis in the 1960s.108 A reference paper stated that to define "chronic," quantitative terms must be introduced into the definition, and at present this must be done more or less arbitrarily, and that the phrase "chronic or recurrent" has usually been accepted as implying that expectoration has occurred on most days during at least three consecutive months for more than 2 successive years.108 This cutoff duration for "chronic" remained in use in later major protocols of the European Community for Coal and Steel (ECSC)-87 and the American Thoracic Society with the Division of Lung Diseases (ATSDLD)-78.109 The ECSC-87 and ATS-DLD-78 questionnaires had modified the BMRC questionnaire by asking for a seasonal association of cough, from in the winter to no specification of season, but not the cutoff duration for chronicity in cough and phlegm. The use of 3 months' cutoff duration has been maintained in recent large-scale population surveys in Europe, including the European Community Respiratory Health Survey (ECRHS) since the 1990s.110
It may be advantageous to utilize these major questionnaires in further population surveys for chronic cough, as they have been extensively validated and have presented little risk for bias from the mode of administration. However, a major dilemma is that the questionnaires were not developed or validated for chronic cough specifically. Moreover, the criterion of 3 months' duration conflicts with the criterion of 8 weeks' duration used in major clinical guidelines for chronic cough in adults.45 However, because neither of them was evidence-based, but rather arose from expert opinions, neither can be viewed as "correct." Before the publication of current clinical guidelines, a ≥3 weeks' duration had often been utilized in clinical studies of chronic or persistent cough,111112113 as common colds were considered to usually resolve within 3 weeks; however, a later consensus was made as 8 weeks114 because post-infectious cough often persists longer than 3 weeks. Meanwhile, in a recent international qualitative study, significant variation was still observed in the definition of chronic cough used in clinical practice, even among specialist clinicians.115 We suggest that the discrepancy on cutoff duration needs to be resolved by prospective studies on the natural course of self-limiting cough and the characteristics of the minority of patients who go on to prolong chronic cough.
As another component, phlegm may need to be discussed. Productive cough could suggest a likelihood of cough associated with infection or smoking. However, it is difficult to quantify or characterize phlegm objectively in large-scale population surveys. Moreover, among chronic cough patients, the history of excessive sputum production did not indicate different natures of cough.113 In our unpublished data, the population-level correlations between current smoking and chronic cough prevalence did not significantly differ between chronic cough subjects with and without phlegm. Collectively, the informative value of classifying chronic cough as dry or productive may be questioned in epidemiological surveys.
Our meta-analysis found a slightly higher proportion of males than females, and a high level of heterogeneity in the sex ratio, among chronic cough subjects in the community. These findings were unexpected, as a homogeneous demographic profile was observed among chronic cough patients visiting specialist clinics, where two-thirds of patients were females.15 The femalepredominant profiles of chronic cough patients are considered plausible, as females have enhanced cough responses to tussigen inhalation compared to males, irrespective of being cough patients or healthy controls.116117118119120
With regard to this discrepancy between community and clinics, socioeconomic or individual factors need to be prospectively investigated first. These may include disease characteristics, health-seeking behaviours, or other non-medical factors. Smoking may be one of these factors; smoking is more prevalent among males than among females (males 40.8%±13.3% vs females 13.7%±17.7% in the present data) and is correlated with chronic cough prevalence, but smokers are less likely to seek medical care for their health problems.121 Moreover, smoker's cough may be resolved by smoking cessation alone,114 also possibly leading to less medical attendance among them. Meanwhile, females have more frequent complications from cough, such as incontinence.122
The discrepancy in sex-specific associations of chronic cough between community and clinics also leads to the speculation that the current duration-based definition does not easily differentiate clinically relevant cough from a protective cough response. As suggested by the cough-smoking correlations,2343753557578 chronic cough among smokers may be a protective response against irritant inhalation. However, chronic cough patients are frequently never smokers122123 and have troublesome cough in response to various trivial triggers, such as cold air, singing/talking, or fatigue/stress,123124125 which represents a hypersensitive cough response. Thus, if we aim to identify subjects with chronic cough that is clinically relevant, we may need to add some components to detect this hypersensitivity. Conventional tussigen inhalation cough reflex tests have limited utility, as there is a significant overlap between chronic cough patients and normal controls.11 It would be ideal to have an objective cough reflex test to define cough hypersensitivity for use in epidemiological surveys.
This study has several limitations. First, our meta-analysis of gender ratio included relatively few studies (n=28; 56%) and thus could not confirm the gender associations of chronic cough prevalence in the community populations. We attempted to contact the corresponding authors by email, but could add sexspecific data in only a few cases. Second, the reasons for the heterogeneity in the pooled gender ratio were not clearly explained by our subgroup analyses. The heterogeneity may arise from the variability in the study designs, protocols, or demographic characteristics of included studies and participants. Among demographic factors, in particular, we suppose age and smoking could influence the gender ratio of chronic cough; however, the lack of available information did not allow us to perform meta-regression tests for smoking, or further subgroup analyses by age groups in detail.
Despite these limitations, this study has strength in that it is the first systematic review on the epidemiological definitions of chronic cough. We had no language restriction in retrieving the relevant publication from major databases, enabling the inclusion of non-English literature and the comprehensive review of existing definitions. Our meta-analysis had explorative nature for the reasons described above, but could identify the unexpected but clear heterogeneity and discrepancy in the gender preponderance between the community and clinics.
In conclusion, this study identified major issues for further epidemiological studies of chronic cough. First, cough ≥3 months' duration was the most common definition, but it conflicts with the criterion of current clinical guidelines (cough ≥8 weeks). Moreover, both criteria were determined by expert opinions rather than clinical evidence. Thus, we may need to develop objective evidence for defining chronicity in cough. Second, we found unexpected discrepancies in the demographic profiles of chronic cough subjects between the community and clinics. This discrepancy needs to be comprehensively explained in further prospective studies, but also may raise a question about the appropriateness of using a duration-based definition for identifying clinically relevant chronic cough.
Figures and Tables
Fig. 2
Time trends in the utilization of definitions in epidemiological studies. (A) Time trends among all articles which reported the prevalence of chronic cough (n=70). (B) Time trends among a subgroup of articles which reported the prevalence of chronic cough as the primary outcome (n=13).
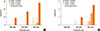
Fig. 3
Forest plots for male-to-female ratio in 28 epidemiological studies using a 'cough ≥3 months' definition and quality effects model. Study name indicates the first author and publication year, and they were ordered according to the study year (the number within the parenthesis). OR, odds ratio; 95% CI, 95% confidence interval.

Table 1
Component of chronic cough definitions utilized in epidemiological studies

| Component | All studies (n=70) | Studies with chronic cough as primary outcome (n=13) |
|---|---|---|
| Duration | ||
| ≥3 months | 19,20,21,23,24,27,28,30,32,33,36,37,38,39,40,41,42,43,44,45,46,47,49,50,52,53,54,55,60,61,62,63,64,65,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,83,84,85,86,87,88 | 19,23,24,30,53,73,81 |
| ≥8 weeks or 2 months | 31,34,35 | 31,34,35 |
| ≥3 weeks | 25 | 25 |
| No specific description | 22,26,29,48,51,56,57,58,59,66,82 | 22,29 |
| Combined phlegm | ||
| Yes (productive cough) | 24,26,47,50,61 | 24 |
| No (dry cough) | 74 | |
| No specific description | 19,20,21,22,23,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,48,49,51,52,53,54,55,56,57,58,59,60,62,63,64,65,66,67,68,69,60,71,72,73,75,76,77,78,79,80,81,82,83,84,85,86,87,88 | 19,22,23,25,29,30,31,34,35,53,73,81 |
| Prevalence measure | ||
| 12-month prevalence | 20,21,23,24,26,27,28,29,30,31,32,33,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,58,60,61,62,63,64,65,67,68,69,70,71,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88 | 23,24,29,30,31,53,73,81 |
| Point prevalence | 19,22,25,34,35,72 | 19,22,25,34,35 |
| No specific description | 56,57,59,66 |
Table 2
Sub-group analyses for sex associations of chronic cough

ACKNOWLEDGMENTS
We gratefully acknowledge the help of GlaxoSmithKline (UK) in providing data translation assistance.
References
1. Morice AH. Epidemiology of cough. Pulm Pharmacol Ther. 2002; 15:253–259.
2. Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015; 45:1479–1481.
3. French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998; 158:1657–1661.
4. Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129:1S–23S.
5. Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004; 24:481–492.
6. Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther. 2003; 17:1515–1520.
7. O'Hara J, Jones NS. "Post-nasal drip syndrome": most patients with purulent nasal secretions do not complain of chronic cough. Rhinology. 2006; 44:270–273.
8. McGarvey LP. Does idiopathic cough exist? Lung. 2008; 186:Suppl 1. S78–S81.
9. Chung KF. Chronic 'cough hypersensitivity syndrome': a more precise label for chronic cough. Pulm Pharmacol Ther. 2011; 24:267–271.
10. Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013; 1:414–422.
11. Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J. 2014; 44:1132–1148.
12. Song WJ, Chang YS, Morice AH. Changing the paradigm for cough: does 'cough hypersensitivity' aid our understanding. Asia Pac Allergy. 2014; 4:3–13.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
14. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013; 67:974–978.
15. Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014; 44:1149–1155.
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
17. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010; 1:97–111.
18. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006; 295:676–680.
19. Adams RJ, Appleton SL, Wilson DH, Taylor AW, Ruffin RE. Associations of physical and mental health problems with chronic cough in a representative population cohort. Cough. 2009; 5:10.
20. Amigo H, Oyarzun MG, Bustos P, Rona RJ. Respiratory consequences of light and moderate smoking in young adults in Chile. Int J Tuberc Lung Dis. 2006; 10:744–749.
21. Bakke P, Eide GE, Hanoa R, Gulsvik A. Occupational dust or gas exposure and prevalences of respiratory symptoms and asthma in a general population. Eur Respir J. 1991; 4:273–278.
22. Bende M, Millqvist E. Prevalence of chronic cough in relation to upper and lower airway symptoms; the Skövde population-based study. Front Physiol. 2012; 3:251.
23. Brown CA, Crombie IK, Smith WC, Tunstall-Pedoe H. The impact of quitting smoking on symptoms of chronic bronchitis: results of the Scottish Heart Health Study. Thorax. 1991; 46:112–116.
24. Cerveri I, Accordini S, Corsico A, Zoia MC, Carrozzi L, Cazzoletti L, et al. Chronic cough and phlegm in young adults. Eur Respir J. 2003; 22:413–417.
25. Chen RC, Lai KF, Liu CL, Luo W, Zhong NS. An epidemiologic study of cough in young college students in Guangzhou. Zhonghua Liu Xing Bing Xue Za Zhi. 2006; 27:123–126.
26. Cheng Y, Guo YL, Yeh WY. A national survey of psychosocial job stressors and their implications for health among working people in Taiwan. Int Arch Occup Environ Health. 2001; 74:495–504.
27. Chhabra P, Sharma G, Kannan AT. Prevalence of respiratory disease and associated factors in an urban area of delhi. Indian J Community Med. 2008; 33:229–232.
28. Comstock GW, Stone RW, Tonascia JA, Johnson DH. Respiratory survey findings as predictors of disability from respiratory diseases. Am Rev Respir Dis. 1981; 124:367–371.
29. Cullinan P. Persistent cough and sputum: prevalence and clinical characteristics in south east England. Respir Med. 1992; 86:143–149.
30. Davydova LI, Khodosh EM, Nikolenko EIa. Prevalence of cough as a predictor and symptom of chronic bronchitis among male population of Kharkov. Klin Med (Mosk). 1991; 69:51–54.
31. Desalu OO, Salami AK, Fawibe AE. Prevalence of cough among adults in an urban community in Nigeria. West Afr J Med. 2011; 30:337–341.
32. Duţu S, Păun G. The prevalence of respiratory symptoms, bronchial asthma and chronic bronchitis (simple and obstructive) in a representative sample of the adult rural population. Pneumoftiziologia. 1998; 47:151–160.
33. Enright PL, Kronmal RA, Higgins MW, Schenker MB, Haponik EF. Prevalence and correlates of respiratory symptoms and disease in the elderly. Cardiovascular Health Study. Chest. 1994; 106:827–834.
34. Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006; 61:975–979.
35. Fujimura M. Frequency of persistent cough and trends in seeking medical care and treatment-results of an internet survey. Allergol Int. 2012; 61:573–581.
36. Gundersen H, Magerøy N, Moen BE, Bråtveit M. Low traffic and respiratory symptoms among smoking females: the Hordaland Health Study. Arch Environ Occup Health. 2012; 67:189–198.
37. Hamzaçebi H, Unsal M, Kayhan S, Bilgin S, Ercan S. Prevalence of asthma and respiratory symptoms by age, gender and smoking behaviour in Samsun, North Anatolia Turkey. Tuberk Toraks. 2006; 54:322–329.
38. Hazenkamp-von Arx ME, Schindler C, Ragettli MS, Künzli N, Braun-Fahrländer C, Liu LJ. Impacts of highway traffic exhaust in alpine valleys on the respiratory health in adults: a cross-sectional study. Environ Health. 2011; 10:13.
39. Hersoug LG, Husemoen LL, Sigsgaard T, Madsen F, Linneberg A. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010; 15:993–1000.
40. Huchon GJ, Vergnenègre A, Neukirch F, Brami G, Roche N, Preux PM. Chronic bronchitis among French adults: high prevalence and underdiagnosis. Eur Respir J. 2002; 20:806–812.
41. Iversen L, Hannaford PC, Price DB, Godden DJ. Is living in a rural area good for your respiratory health? Results from a cross-sectional study in Scotland. Chest. 2005; 128:2059–2067.
42. Jaén A, Zock JP, Kogevinas M, Ferrer A, Marín A. Occupation, smoking, and chronic obstructive respiratory disorders: a cross sectional study in an industrial area of Catalonia, Spain. Environ Health. 2006; 5:2.
43. Korn RJ, Dockery DW, Speizer FE, Ware JH, Ferris BG Jr. Occupational exposures and chronic respiratory symptoms. A population-based study. Am Rev Respir Dis. 1987; 136:298–304.
44. Krzyzanowski M, Kauffmann F. The relation of respiratory symptoms and ventilatory function to moderate occupational exposure in a general population. Results from the French PAARC study of 16,000 adults. Int J Epidemiol. 1988; 17:397–406.
45. Krzyzanowski M, Wysocki M. The relation of thirteen-year mortality to ventilatory impairment and other respiratory symptoms: the Cracow Study. Int J Epidemiol. 1986; 15:56–64.
46. Kumar R, Sharma M, Srivastva A, Thakur JS, Jindal SK, Parwana HK. Association of outdoor air pollution with chronic respiratory morbidity in an industrial town in northern India. Arch Environ Health. 2004; 59:471–477.
47. Lai CK, Ho SC, Lau J, Yuen YK, Ho SS, Chan CH, et al. Respiratory symptoms in elderly Chinese living in Hong Kong. Eur Respir J. 1995; 8:2055–2061.
48. Lâm HT, Rönmark E, Tu’ò’ng NV, Ekerljung L, Chúc NT, Lundbäck B. Increase in asthma and a high prevalence of bronchitis: results from a population study among adults in urban and rural Vietnam. Respir Med. 2011; 105:177–185.
49. Lebowitz MD. Multivariate analysis of smoking and other risk factors for obstructive lung diseases and related symptoms. J Chronic Dis. 1982; 35:751–758.
50. Lindström M, Kotaniemi J, Jönsson E, Lundbäck B. Smoking, respiratory symptoms, and diseases : a comparative study between northern Sweden and northern Finland: report from the FinEsS study. Chest. 2001; 119:852–861.
51. Lötvall J, Ekerljung L, Rönmark EP, Wennergren G, Lindén A, Rönmark E, et al. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res. 2009; 10:94.
52. Lundbäck B, Nyström L, Rosenhall L, Stjernberg N. Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J. 1991; 4:257–266.
53. Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijayasimha R. Prevalence of chronic cough, chronic phlegm & associated factors in Mysore, Karnataka, India. Indian J Med Res. 2011; 134:91–100.
54. Matsuda S, Nguyen AL, Jonai H, Nguyen VH, Dinh HT, Le VT, et al. Occupational exposure and chronic respiratory symptoms--a population based study in Vietnam. Ind Health. 1997; 35:271–277.
55. Mensinga TT, Schouten JP, Rijcken B, Weiss ST, Speizer FE, van der Lende R. The relationship of eosinophilia and positive skin test reactivity to respiratory symptom prevalence in a communitybased population study. J Allergy Clin Immunol. 1990; 86:99–107.
56. Milenković B, Mitić-Milić M, Rebić P, Vukcević M, Dudvarski-Ilić A, Nagorni-Obradović L, et al. Asthma and chronic bronchitis symptoms among adult population of Belgrade. Srp Arh Celok Lek. 2011; 139:149–154.
57. Mirabelli MC, London SJ, Charles LE, Pompeii LA, Wagenknecht LE. Occupation and the prevalence of respiratory health symptoms and conditions: the Atherosclerosis Risk in Communities Study. J Occup Environ Med. 2012; 54:157–165.
58. Montnémery P, Bengtsson P, Elliot A, Lindholm LH, Nyberg P, Löfdahl CG. Prevalence of obstructive lung diseases and respiratory symptoms in relation to living environment and socio-economic group. Respir Med. 2001; 95:744–752.
59. Mossakowska M, Pawlinska-Chmara R, Broczek KM. Asthma, allergy, and respiratory symptoms in centenarians living in Poland. J Physiol Pharmacol. 2008; 59:Suppl 6. 483–489.
60. Norboo T, Yahya M, Bruce NG, Heady JA, Ball KP. Domestic pollution and respiratory illness in a Himalayan village. Int J Epidemiol. 1991; 20:749–757.
61. Nordenstedt H, Nilsson M, Johansson S, Wallander MA, Johnsen R, Hveem K, et al. The relation between gastroesophageal reflux and respiratory symptoms in a population-based study: the Nord-Trøndelag health survey. Chest. 2006; 129:1051–1056.
62. Nriagu J, Robins T, Gary L, Liggans G, Davila R, Supuwood K, et al. Prevalence of asthma and respiratory symptoms in south-central Durban, South Africa. Eur J Epidemiol. 1999; 15:747–755.
63. Omenaas E, Fluge O, Buist AS, Vollmer WM, Gulsvik A. Dietary vitamin C intake is inversely related to cough and wheeze in young smokers. Respir Med. 2003; 97:134–142.
64. Pallasaho P, Lundbäck B, Meren M, Kiviloog J, Loit HM, Larsson K, et al. Prevalence and risk factors for asthma and chronic bronchitis in the capitals Helsinki, Stockholm, and Tallinn. Respir Med. 2002; 96:759–769.
65. Peat JK, Haby M, Spijker J, Berry G, Woolcock AJ. Prevalence of asthma in adults in Busselton, Western Australia. BMJ. 1992; 305:1326–1329.
66. Purty AJ, Bazroy J, Kar M, Vasudevan K, Veliath A, Panda P. Morbidity pattern among the elderly population in the rural area of Tamil Nadu, India. Turk J Med Sci. 2006; 36:45–50.
67. Rijcken B, Schouten JP, Weiss ST, Speizer FE, van der Lende R. The relationship of nonspecific bronchial responsiveness to respiratory symptoms in a random population sample. Am Rev Respir Dis. 1987; 136:62–68.
68. Samet JM, Schrag SD, Howard CA, Key CR, Pathak DR. Respiratory disease in a New Mexico population sample of Hispanic and non-Hispanic whites. Am Rev Respir Dis. 1982; 125:152–157.
69. Schenker MB, Samet JM, Speizer FE. Effect of cigarette tar content and smoking habits on respiratory symptoms in women. Am Rev Respir Dis. 1982; 125:684–690.
70. Shin C, Lee S, Abbott RD, Kim JH, Lee SY, In KH, et al. Respiratory symptoms and undiagnosed airflow obstruction in middle-aged adults: the Korean Health and Genome Study. Chest. 2004; 126:1234–1240.
71. Skorge TD, Eagan TM, Eide GE, Gulsvik A, Bakke PS. Indoor exposures and respiratory symptoms in a Norwegian community sample. Thorax. 2005; 60:937–942.
72. Sobradillo V, Miravitlles M, Jiménez CA, Gabriel R, Viejo JL, Masa JF, et al. Epidemiological study of chronic obstructive pulmonary disease in Spain (IBERPOC): prevalence of chronic respiratory symptoms and airflow limitation. Arch Bronconeumol. 1999; 35:159–166.
73. Song WJ, Morice AH, Kim MH, Lee SE, Jo EJ, Lee SM, et al. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013; 8:e78081.
74. Songür N, Aydin ZD, Oztürk O, Sahin U, Khayri U, Bircan A, et al. Respiratory symptoms, pulmonary function, and reproductive history: Isparta Menopause and Health Study. J Womens Health (Larchmt). 2010; 19:1145–1154.
75. Tollerud DJ, O'Connor GT, Sparrow D, Weiss ST. Asthma, hay fever, and phlegm production associated with distinct patterns of allergy skin test reactivity, eosinophilia, and serum IgE levels. The Normative Aging Study. Am Rev Respir Dis. 1991; 144:776–781.
76. Turcić N, Zuskin E, Mustajbegović J, Smolej-Narancić N, Ivanković D. Respiratory symptoms, diseases and pulmonary ventilatory capacity in persons in the third stage of life. Lijec Vjesn. 2002; 124:247–254.
77. Venners SA, Wang B, Ni J, Jin Y, Yang J, Fang Z, et al. Indoor air pollution and respiratory health in urban and rural China. Int J Occup Environ Health. 2001; 7:173–181.
78. Viegi G, Paoletti P, Carrozzi L, Vellutini M, Diviggiano E, Di Pede C, et al. Prevalence rates of respiratory symptoms in Italian general population samples exposed to different levels of air pollution. Environ Health Perspect. 1991; 94:95–99.
79. Viegi G, Paoletti P, Prediletto R, Carrozzi L, Fazzi P, Di Pede F, et al. Prevalence of respiratory symptoms in an unpolluted area of northern Italy. Eur Respir J. 1988; 1:311–318.
80. Voll-Aanerud M, Eagan TM, Plana E, Omenaas ER, Bakke PS, Svanes C, et al. Respiratory symptoms in adults are related to impaired quality of life, regardless of asthma and COPD: results from the European community respiratory health survey. Health Qual Life Outcomes. 2010; 8:107.
81. Walraven GE, Nyan OA, Van Der Sande MA, Banya WA, Ceesay SM, Milligan PJ, et al. Asthma, smoking and chronic cough in rural and urban adult communities in The Gambia. Clin Exp Allergy. 2001; 31:1679–1685.
82. Wang JH, Luo JY, Dong L, Gong J, Tong M. Epidemiology of gastroesophageal reflux disease: a general population-based study in Xi'an of Northwest China. World J Gastroenterol. 2004; 10:1647–1651.
83. Wang X, Deng FR, Lv HB, Wu SW, Guo XB. Long-term effects of air pollution on the occurrence of respiratory symptoms in adults of Beijing. Beijing Da Xue Xue Bao. 2011; 43:356–359.
84. Wilson D, Takahashi K, Pan G, Chan CC, Zhang S, Feng Y, et al. Respiratory symptoms among residents of a heavy-industry province in China: prevalence and risk factors. Respir Med. 2008; 102:1536–1544.
85. Xu X, Christiani DC, Dockery DW, Wang L. Exposure-response relationships between occupational exposures and chronic respiratory illness: a community-based study. Am Rev Respir Dis. 1992; 146:413–418.
86. Zemp E, Elsasser S, Schindler C, Künzli N, Perruchoud AP, Domenighetti G, et al. The SAPALDIA Team. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). Am J Respir Crit Care Med. 1999; 159:1257–1266.
87. Zhang LX, Enarson DA, He GX, Li B, Chan-Yeung M. Occupational and environmental risk factors for respiratory symptoms in rural Beijing, China. Eur Respir J. 2002; 20:1525–1531.
88. Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997; 350:85–90.
89. Accordini S, Corsico AG, Cerveri I, Antonicelli L, Attena F, Bono R, et al. Diverging trends of chronic bronchitis and smoking habits between 1998 and 2010. Respir Res. 2013; 14:16.
90. Burr ML, Holliday RM. Why is chest disease so common in South Wales? Smoking, social class, and lung function: a survey of elderly men in two areas. J Epidemiol Community Health. 1987; 41:140–144.
91. Cetinkaya F, Gülmez I, Aydin T, Oztürk Y, Ozesmi M, Demir R. Prevalence of chronic bronchitis and associated risk factors in a rural area of Kayseri, Central Anatolia, Turkey. Monaldi Arch Chest Dis. 2000; 55:189–193.
92. Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001; 164:372–377.
93. de Oca MM, Halbert RJ, Lopez MV, Perez-Padilla R, Tálamo C, Moreno D, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012; 40:28–36.
94. Desalu OO, Salami AK, Seidu OA, Olokoba AB, Fadeyi A. Factors associated with nocturnal, productive and dry cough in the young adult population of Nigeria. J Bras Pneumol. 2010; 36:325–331.
95. Duţu S, Jienescu Z, Băicoianu S. Epidemiological study of chronic bronchitis in a population sample aged 60-85 years: contributions to the study of the natural history of chronic bronchitis (author's transl). Respiration. 1980; 39:49–59.
96. Ehrlich RI, White N, Norman R, Laubscher R, Steyn K, Lombard C, et al. Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis. 2004; 8:369–376.
97. Freitas FT, Yokota RT, de Castro AP, Andrade SS, Nascimento GL, de Moura NF, et al. Prevalence of respiratory symptoms in areas of the Federal District, Brazil. Rev Panam Salud Publica. 2011; 29:451–456.
98. Frostad A, Søyseth V, Andersen A, Gulsvik A. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med. 2006; 259:520–529.
99. Golshan M, Faghihi M, Marandi MM. Indoor women jobs and pulmonary risks in rural areas of Isfahan, Iran, 2000. Respir Med. 2002; 96:382–388.
100. Grievink L, Smit HA, Ocké MC, van 't Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998; 53:166–171.
101. Jakeways N, McKeever T, Lewis SA, Weiss ST, Britton J. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur Respir J. 2003; 21:658–663.
102. Ko FW, Lai CK, Woo J, Ho SC, Ho CW, Goggins W, et al. 12-year change in prevalence of respiratory symptoms in elderly Chinese living in Hong Kong. Respir Med. 2006; 100:1598–1607.
103. Littlejohns P, Ebrahim S, Anderson R. Prevalence and diagnosis of chronic respiratory symptoms in adults. BMJ. 1989; 298:1556–1560.
104. Pertuzé J, Contreras G, Deck C, Ferretti R, Lisboa C, Cruz E, et al. Respiratory symptoms, pulmonary function and the smoking habit in urban and rural areas of Chile. Rev Med Chil. 1986; 114:993–999.
105. von Hertzen L, Reunanen A, Impivaara O, Mälkiä E, Aromaa A. Airway obstruction in relation to symptoms in chronic respiratory disease--a nationally representative population study. Respir Med. 2000; 94:356–363.
106. Wheaton AG, Ford ES, Thompson WW, Greenlund KJ, Presley-Cantrell LR, Croft JB. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the United States--National Health and Nutrition Examination Survey 2007-2010. BMC Public Health. 2013; 13:854.
107. Wicht CL, Kotze TJ. The influence of smoking habits on the symptoms of diffuse obstructive pulmonary syndrome. S Afr Med J. 1981; 60:26–30.
108. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965; 1:775–779.
109. Liard R, Neukirch F. Questionnaires: a major instrument for respiratory epidemiology. Eur Respir Monogr. 2000; 15:154–166.
110. Pistelli F, Maio S. Questionnaires and lung function. ERS Monogr. 2014; 65:257–272.
111. McGarvey LP, Heaney LG, Lawson JT, Johnston BT, Scally CM, Ennis M, et al. Evaluation and outcome of patients with chronic nonproductive cough using a comprehensive diagnostic protocol. Thorax. 1998; 53:738–743.
112. Mello CJ, Irwin RS, Curley FJ. Predictive values of the character, timing, and complications of chronic cough in diagnosing its cause. Arch Intern Med. 1996; 156:997–1003.
113. Smyrnios NA, Irwin RS, Curley FJ. Chronic cough with a history of excessive sputum production. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest. 1995; 108:991–997.
114. Irwin RS, Boulet LP, Cloutier MM, Fuller R, Gold PM, Hoffstein V, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest. 1998; 114:133S–181S.
115. Faruqi S, Murdoch RD, Allum F, Morice AH. On the definition of chronic cough and current treatment pathways: an international qualitative study. Cough. 2014; 10:5.
116. Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1998; 113:1319–1321.
117. Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Sex difference in the inhaled tartaric acid cough threshold in non-atopic healthy subjects. Thorax. 1990; 45:633–634.
118. Kastelik JA, Thompson RH, Aziz I, Ojoo JC, Redington AE, Morice AH. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med. 2002; 166:961–964.
119. Kelsall A, Decalmer S, McGuinness K, Woodcock A, Smith JA. Sex differences and predictors of objective cough frequency in chronic cough. Thorax. 2009; 64:393–398.
120. Rostami-Hodjegan A, Abdul-Manap R, Wright CE, Tucker GT, Morice AH. The placebo response to citric acid-induced cough: pharmacodynamics and gender differences. Pulm Pharmacol Ther. 2001; 14:315–319.
121. Jorm LR, Shepherd LC, Rogers KD, Blyth FM. Smoking and use of primary care services: findings from a population-based cohort study linked with administrative claims data. BMC Health Serv Res. 2012; 12:263.
122. Everett CF, Kastelik JA, Thompson RH, Morice AH. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007; 3:5.
123. Song WJ, Kim JY, Jo EJ, Lee SE, Kim MH, Yang MS, et al. Capsaicin cough sensitivity is related to the older female predominant feature in chronic cough patients. Allergy Asthma Immunol Res. 2014; 6:401–408.
124. Matsumoto H, Tabuena RP, Niimi A, Inoue H, Ito I, Yamaguchi M, et al. Cough triggers and their pathophysiology in patients with prolonged or chronic cough. Allergol Int. 2012; 61:123–132.
125. Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011; 189:73–79.
Appendix
Appendix Table S1
Characteristics of 70 studies which reported the prevalence of chronic, longstanding, or persistent cough in general adult populations
Appendix Table S4
Classification of definitions according to terminology and question among 70 epidemiological studies for chronic cough
Appendix Fig. S1
Forest plots for male-to-female ratio in 28 epidemiological studies using a 'cough ≥3 months' definition and random effects model. Study name indicates the first author and publication year, and they were ordered according to the study year (the number within the parenthesis). OR, odds ratio; 95% CI, 95% confidence interval.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download