Abstract
Purpose
The cockroach (CR) is an important cause of respiratory allergic disorders. We prepared a German CR extract in a standardized way and analyzed its allergenic properties.
Methods
The extract was prepared from German CR (Blattella germanica) obtained from a Korean colony, and its allergenic activity was compared with that of the commercial Hollister-Stier (HS) extract. The concentrations of Bla g 1 and Bla g 2 were measured, and an in vitro specific IgE binding inhibition assay was performed to assess IgE reactivity. Proteolytic activity was examined by gelatin zymography.
Results
Bla g 1 and Bla g 2 were detected at 405 U/mg and 273 ng/mg, respectively, in the Korean extract, and at 187 U/mg and 56 ng/mg, respectively, in the HS extract. The Korean extract showed 94.2% inhibition of IgE reactivity, as compared with the HS extract. A similar pattern of IgE-reactive bands was detected for the two extracts, indicating that their allergenic components are similar. The proteolytic activities of the Korean and HS extracts were found to be similar in gelatin zymography. The endotoxin levels in the Korean and HS extracts were 3,440 EU/mL and 6,580 EU/mL, respectively.
Cockroach (CR) is an important source of inhalant allergens, and sensitization to CR is associated with asthma exacerbation.1 Moreover, epidemiologic data show a close relationship between CR sensitization and the prevalence of allergic asthma.2 Standardization of allergen extracts is important for diagnosis and immunotherapy.3 The German CR Blattella germanica is the most commonly found CR in Korean homes.4,5 However, CR extracts have not been standardized. The levels of protein and major allergens (Bla g 1 and Bla g 2) vary in the CR extracts that are commercially available in the USA.6 The concentration of CR extract supplied by the manufacturers is usually expressed in weight to volume (w/v) units. A designation of 1:10 w/v indicates that the solution contains the extractable material from 1 g of raw material added to 10 mL of buffer solution. The biological potencies of commercial German CR extracts have been estimated at 10-8570 bioequivalent allergy units (BAU)/mL in the USA.6,7 Moreover, protease activity is known to play an important role in the pathogenesis of CR allergy.8 CR extracts contain various proteases, which can degrade proteins, including allergens, in the extracts.9 Therefore, it is desirable to produce CR extracts that retain considerable protease activity without any significant degradation of the IgE-reactive components.
In the present study, we used a standardized method to produce extracts of German CRs, which were reared at the Korea National Arthropods of Medical Importance Resource Bank, Yonsei University College of Medicine, Seoul, Korea. The concentrations of the major allergens (Bla g 1 and Bla g 2) in the Korean extracts were compared with those of an extract obtained from a US company, i.e., the Hollister-Stier (HS) extract. The allergenic activities of these extracts were compared in an in vitro inhibition analysis.
Lyophilized CR (20 g) was pulverized using a mortar and pestle. The powdered CR (10 g) was defatted with ethyl ether (1:5, w/v). The allergen was extracted for 48 hours at 4℃ in phosphate-buffered saline (PBS; pH 7.4) that contained 0.2% phenol. The extract was centrifuged at 13,000 ×g for 15 minutes at 4℃, and the supernatant was dialyzed (cut-off, 3,500 Da; Spectrum, Houston, TX, USA) extensively against distilled water. The dialyzed sample was filtered (0.22-µm pore; Millipore, Bedford, MA, USA) and lyophilized once again. The protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA) after reconstitution in buffers. Thereafter, the extract was aliquoted, lyophilized, and stored at -80℃ until use.
The protein profiles of the CR extracts were examined by SDS-PAGE. Samples (10 µg), which were reconstituted in PBS (pH 7.4) that contained 50% glycerol and 0.03% human serum albumin (HSA), were run on 12.5% gels under reducing conditions. Proteins were visualized by staining with Coomassie Brilliant Blue R250 or transferred onto nitrocellulose membrane (Amersham, Buckinghamshire, UK). The membranes were incubated with 1:4 dilutions of sera (pooled serum from five patients or five healthy controls) after blocking with 3% skim milk in TBST (50 mM Tris [pH 7.5], 0.05% Tween-20). Subsequently, IgE-reactive proteins were probed with alkaline phosphatase-conjugated goat anti-human IgE (1:1,000; Sigma-Aldrich, St. Louis, MO, USA) for 1 hour, and the color was developed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Promega, Madison, WI, USA). The membrane was washed three times with TBST between each incubation step.
Allergen potencies were compared using the competitive specific IgE binding CAP inhibition system with the HS allergen extract (Hollister-Stier Laboratories, Spokane, WA, USA). The Korean extract dissolved in distilled water was used for the inhibition study. The serum samples (diluted 1:4) were pre-incubated with various concentrations of inhibitors (0.001 µg/mL to 20 µg/mL), and anti-human IgE reactivity was measured using the UniCAP system (Phadia, Uppsala, Sweden), according to the manufacturer's instruction. The percentage of inhibition was calculated as: (1-Ai/A0)×1,000, where Ai is the IgE value (kU/mL) with inhibitor and A0 is the IgE value without inhibitor.
The levels of the CR allergens, Bla g 1 and Bla g 2, in the CR extracts reconstituted in distilled water were determined by two-site ELISA (Indoor Biotechnologies Inc., Charlottesvillle, VA, USA). Endotoxin content was measured using QCL-1000 (Lonza, Walkersville, MD, USA).
For the analysis of gelatinolytic proteases, samples (200 ng/well), which were reconstituted in PBS (pH 7.4) that contained 50% glycerol and 0.03% HSA, were run on 10% SDS-PAGE gels that contained 0.1% gelatin (Invitrogen). After electrophoresis, the gel was incubated in renaturing buffer (Invitrogen) for 1 hour at room temperature, and then placed in developing buffer (Invitrogen) overnight at 4℃. The gel was stained with Coomassie Brilliant Blue R250.
The protein concentration of the Korean extract was very low (410 µg/mL) compared with that of the HS extract (2,300 µg/mL). However, the concentrations of Bla g 1 and Bla g 2 in the Korean extract (405 U/mg and 273 ng/mg, respectively) were higher than those in the HS extract (187 U/mg and 56 ng/mg, respectively) (Table).
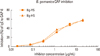
The allergenic activities of the CR extracts were examined using an in vitro specific IgE binding inhibition assay. The Korean extract showed 94.2% inhibition of IgE reactivity, as compared with the HS extract (Fig. 2).
The Korean extract had more apparent bands in the SDS-PAGE analysis (Fig. 1A). The presence of a protein band close to the well in the HS extract lane implies some aggregation of the extract. No IgE-reactive bands were detected when sera from healthy controls were used for the immunoblotting (data not shown).
A broad range of gelatinolytic activity was detected for the proteins in the range of 10-100 kDa in both the Korean and HS extracts (Fig. 1C). In both extracts, the primary proteolytic activity was detected for proteins of 10-30 kDa.
The endotoxin levels of the Korean and HS extracts were determined to be 3,440 EU/mL and 6,580 EU/mL, respectively (Table). However, the endotoxin level per mg of protein was much higher in the Korean extract (8,390 EU/mg) than in the HS extract (2,861 EU/mg).
We produced a German CR extract using a standardized procedure and characterized the extract by comparing it with the HS extract.
In the electrophoretic analysis, the Korean extract showed multiple bands with molecular masses that ranged from 15 kDa to 100 kDa, including the 66-kDa band of HSA, which is generally added to allergen extracts to increase stability (Fig. 1A). However, the HS extract had less-pronounced bands and some aggregation of proteins around the well. The overall patterns of IgE-reactive proteins in the IgE immunoblots were similar for the Korean and HS extracts, with the exception of the aggregated proteins (Fig. 1B). The proteolytic activities of the two extracts, as revealed by gelatin zymography, were similar (Fig. 1C). The Korean German CR extract was able to inhibit 94.2% of the IgE reactivity, as compared with the HS extract (Fig. 2). Overall allergenicity is essentially identical for the extracts from HS and Korea. Further characterization of the allergenicities of the extracts using an in vivo method is needed.
The levels of Bla g 1 and Bla g 2 in the two extracts were found to be quite different (Table). Polymorphisms of mite allergen sequences are thought to influence the amount of allergens in mite extracts.10 However, no sequence variability was found for the recognition sites on Bla g 2 for the monoclonal antibodies 7C11 and 4C3 (capture and detection antibodies for Bla g 2; Indoor Biotechnologies Inc.).11
Bla g 1 (25-30 kDa) and Bla g 2 (36 kDa) have not been confirmed as the major IgE binding proteins in Korean patients with allergic diseases.12 The IgE reactivities of purified Bla g 1 and Bla g 2 have been described as not being strong,13,14 and the major allergens that dominate the allergenicity of the CR extract need to be identified. Components with molecular masses of less than 14, 38, 50, 64, and 76 kDa have been described as important in Korea.12 The Bla g 2 levels of the extracts were found to be non-proportional to the overall allergenicities.7 It is very interesting to observe strongly IgE-reactive proteins of approximately 60 kDa and 70 kDa in both the Korean and HS extracts (Fig. 1B). The observed similarity of the patterns of IgE-reactive bands in the immunoblots indicates the presence of similar IgE-reactive components in the extracts. Identification of these allergens, which are important in Korea, is necessary for enhanced characterization and standardization of the CR extract.
CR extract is rich in proteases,9 which can activate the various cell types that are important in allergic airway inflammation.15 Special care is needed, although not recommended, when combining fungal and CR extracts for immunotherapy, given the stability of the allergens in the mixtures.16
High levels of endotoxin were detected in the CR extracts, as compared to house dust mite extracts (1-8,485 EU/mL).17 The average levels of endotoxin detected in standardized house dust mite extracts were: 4,619 EU/mL (range, 849-8,485 EU/mL) for seven Dermatophagoides farinae extracts; and 11 EU/mL (range, 1-34 EU/mL) for seven D. pteronyssinus extracts. However, up to 33,805 EU/mL of endotoxin was detected in the standardized cat pelt extract.17 CRs are known to carry various microorganisms, including bacteria.18,19 The endotoxin present in the CR extract is believed to be mainly derived from the flora or commensals in the CR gut.
The German CR extract prepared in the present study could be useful for the development of allergy diagnostics and immunotherapeutics in Korea.
Figures and Tables
Fig. 1
Protein analysis of the German cockroach extracts. Allergen extracts (10 µg/well) were separated on a 12% SDS-PAGE gel under reducing conditions (A). The allergenic components of the extracts were compared by IgE immunoblotting (B). Gelatinolytic protease activity was analyzed on a 10% SDS-PAGE gel (0.2 µg/well) that contained 0.1% gelatin (C). M, molecular mass marker; K, Korean German cockroach extract; HS, US German cockroach extract (Hollister-Stier).
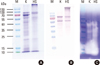
Fig. 2
Inhibition analysis of the cockroach extracts. The specific IgE antibody binding activities of the German cockroach extracts prepared in Korea (Bg-YS) and the US (Bg-HS) were compared in an in vitro specific IgE antibody binding inhibition assay.
Table
Concentration of major allergen and endotoxin in German cockroach extracts

ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A092076).
References
1. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997. 336:1356–1363.
2. Ahluwalia SK, Matsui EC. The indoor environment and its effects on childhood asthma. Curr Opin Allergy Clin Immunol. 2011. 11:137–143.
3. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011. 52:393–400.
4. Kwon SW, Oh SG, Yoon UK, Kim SW, Lee YS, Kim KH, Kim WJ, Kim JK, Kim DS, Kim HS, Ryu IS, Ree SY, Jeaung BJ, Kim KE, Kim DS, Lee KY, Lee HI. Distribution of cockroaches in Korea. Allergy. 1993. 13:334–341.
5. Jeong KY, Lee IY, Lee J, Ree HI, Hong CS, Yong TS. Effectiveness of education for control of house dust mites and cockroaches in Seoul, Korea. Korean J Parasitol. 2006. 44:73–79.
6. Patterson ML, Slater JE. Characterization and comparison of commercially available German and American cockroach allergen extracts. Clin Exp Allergy. 2002. 32:721–727.
7. Slater JE, James R, Pongracic JA, Liu AH, Sarpong S, Sampson HA, Satinover SM, Woodfolk JA, Mitchell HE, Gergen PJ, Eggleston PA. Biological potency of German cockroach allergen extracts determined in an inner city population. Clin Exp Allergy. 2007. 37:1033–1039.
8. Wada K, Matsuwaki Y, Moriyama H, Kita H. Cockroach induces inflammatory responses through protease-dependent pathways. Int Arch Allergy Immunol. 2011. 155:Suppl 1. 135–141.
9. Jeong KY, Kim C, Yong TS. Enzymatic activities of allergen extracts from three species of dust mites and cockroaches commonly found in Korean home. Korean J Parasitol. 2010. 48:151–155.
10. Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, Lee JS, Hong CS, Park JW. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res. 2012. 4:346–350.
11. Jeong KY, Lee H, Shin KH, Yi MH, Jeong KJ, Hong CS, Yong TS. Sequence polymorphisms of major German cockroach allergens Bla g 1, Bla g 2, Bla g 4, and Bla g 5. Int Arch Allergy Immunol. 2008. 145:1–8.
12. Lee SY, Kim DS, Kim KE, Jeaung BJ, Lee KY. IgE binding patterns to German cockroach whole body extract in Korean atopic asthmatic children. Yonsei Med J. 1998. 39:409–416.
13. Yi MH, Jeong KY, Kim CR, Yong TS. IgE-binding reactivity of peptide fragments of Bla g 1.02, a major German cockroach allergen. Asian Pac J Allergy Immunol. 2009. 27:121–129.
14. Lee H, Jeong KY, Shin KH, Yi MH, Gantulaga D, Hong CS, Yong TS. Reactivity of German cockroach allergen, Bla g 2, peptide fragments to IgE antibodies in patients' sera. Korean J Parasitol. 2008. 46:243–246.
15. Page K. Role of cockroach proteases in allergic disease. Curr Allergy Asthma Rep. 2012. 12:448–455.
16. Grier TJ, LeFevre DM, Duncan EA, Esch RE, Coyne TC. Allergen stabilities and compatibilities in mixtures of high-protease fungal and insect extracts. Ann Allergy Asthma Immunol. 2012. 108:439–447.
17. Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003. 111:777–783.
18. Baumholtz MA, Parish LC, Witkowski JA, Nutting WB. The medical importance of cockroaches. Int J Dermatol. 1997. 36:90–96.
19. Tatfeng YM, Usuanlele MU, Orukpe A, Digban AK, Okodua M, Oviasogie F, Turay AA. Mechanical transmission of pathogenic organisms: the role of cockroaches. J Vector Borne Dis. 2005. 42:129–134.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download