Abstract
Atopic dermatitis (AD) is a complex disease that affects up to 20% of children and impacts the quality of patients and families in a significant manner. New insights into the pathophysiology of AD point to an important role of structural abnormalities in the epidermis combined with immune dysregulation. Filaggrin (FLG) is synthesized as a large precursor, profilaggrin, and is expressed in the upper layers of the epidermis. FLG plays a critical role in the epidermal barrier, and FLG mutations cause abnormal epidermal function. FLG mutations are strongly associated with early-onset, and persistent severe AD. In addition, FLG deficiency in the epidermis is related to allergic sensitization and asthma. The basic skin care including repair and protection of the skin barrier with proper hydration and topical anti-inflammatory therapy is important to control the severity of skin disease in patients with AD.
Atopic dermatitis (AD) is a common chronic inflammatory skin disease affecting 15-25% of children and 3% among adults.1-3 It is strongly associated with asthma and allergic sensitization.1-3 Recent data showed that AD is a major problem in developing, as well as developed countries.1 Approximately 85% of patients with AD begin during early childhood, and 70% of patients severe AD develop asthma or allergic rhinitis later in life.4-6 Patients with severe or persistent AD and their families suffer from significant impairment in their quality of life.7 In addition, AD places a heavy economic burden not only on patients and their families, but also on society in general.8,9 AD skin is characterized by immune dysregulation and epidermal barrier defects such as abnormal terminal differentiation of keratinocytes and decreased cornification.6,10-12 This review will focus on structural abnormalities in the epidermis and their modulation by immune responses.
The epidermis provides a physical and permeability barrier (Figure).13-15 This barrier is continuously regenerated by terminally differentiating keratinocytes, a process known as cornification or keratinization. Epidermal differentiation begins with the migration of keratinocytes from the basal layer, and ends with the formation of the cornified layer.13,14 Human epidermis undergoes complete turnover every 28 days.16 Cell proliferation, differentiation and death occur sequentially, and each process is characterized by the expression of specific proteins including Filaggrin (FLG), Loricrin (LOR) and Involucrin (IVL) that are cross linked to form a impermeable skin barrier.13
Profilaggrin is a member of the fused S100 family of S100 Ca2+-binding proteins and a large (-500 kDa) complex protein.13,14 It is expressed in the granular layer of epidermis and consists of a unique N-terminal domain; a region with multiple FLG repeats; and a unique C-terminal end domain.13,14 During terminal differentiation at the granular to cornified cell transition, profilaggrin is rapidly dephosphorylated and cleaved by several endoproteases including caspase-14, to generate FLG and the N-terminal domain.13,17,18 FLG aggregates the keratin filaments into tight bundles.13,14 FLG proteolysis occurs upon exposure to a low humidity environment and might be inhibited by high humidity.19 In the cornified cell, FLG is degraded into free amino acids by caspase-14 and these amino acids are essential for the retention of water contributing to the osmolarity in the cornified layer.17,18
LOR is expressed in the granular layer during cornification and an insoluble protein.13,14 LOR is also one of the main components of the epidermal envelope and is intermixed with profilaggrin, comprising 80% of the total protein mass of the cornified layer.20 LOR seems to function as a major reinforcement protein for the cornified envelope.14,21 IVL is a common component of the cornified envelope and consists of repeating peptide units.22 IVL is an early component in the assembly of cornified envelops and provides a scaffold to which other proteins subsequently become crosslinked.14 In the cornified-envelope structure, IVL is adjacent to the cell membrane.14
The epidermis serves as the first line of defense against invading pathogens and allergens.3,23,24 The epidermal cornified layer is 15 to 29 nm thick and composed of structural proteins and a specialized lipid layer.25,26 Various investigators have demonstrated that a defective skin barrier enhances allergen sensitization, leading to systemic allergic responses such as increased IgE levels and airway hyper-reactivity.27,28 FLG deficiency alters the shape of corneocytes in the human skin,12 and exhibits skin inflammation and enhances epicutaneous sensitization in murine models of eczema.15 In addition, it has been shown that FLG deficiency confers a paracellular skin barrier abnormality that reduces inflammatory thresholds to irritants and haptens.12
On the other hand, the absence of LOR in null mice results in no obvious abnormality, although an early, temporary deficiency in water-barrier function is observed.29 This indicates that keratinocytes could compensate for the loss of LOR by using other available proteins to form a functional cornified layer.30 The specialized lipid layer in the conrnified envelope also provide a barrier against water and prevent water loss.11,12,26
FLG mutations are the best known causes of impaired skin barrier and is considered as predisposing factors for AD.2,31,32 Many genes have been associated with AD and these genes include components of the epidermal skin barrier.33 R510X and 2282del4 are the most common mutant alleles associated with AD in Northern European populations with 10 to 50% of patients with AD having 1 of 2 loss-of-function mutations in FLG.11,34-37 The FLG mutations are currently considered as a major risk factor for AD, particularly in patients who have onset of AD at 2 years or younger and patients with persistent AD.31,38 It has been reported that an individual having one FLG null mutation is at four-fold greater risk of having early-onset AD, while an individual with two mutant FLG allels has an ~80-fold increased risk of developing AD compared with an individual with normal FLG alleles.39 The FLG null alleles identified in asian populations are also significant predisposing factors for AD.40,41
However, it is likely that aside from FLG mutations, there will like be other causes of epidermal barrier defects in AD. In this regard, a significant number of patients with AD do not have any of the known FLG mutations, and conversely, approximately 40% of individuals with FLG-null alleles do not develop AD.37 In addition, up to 50% of patients with AD have FLG mutations, and those who have the mutation eventually recover from the disease.42 Therefore, more work is needed to identify other causes of epidermal abnormalities in AD beyond FLG mutations, and why some individuals never get AD despite the fact that they carry these mutations.
The pathophysiology of AD is not well understood, although it is clear that gene-environment interactions in genetically predisposed individuals play a central role. Investigators have shown a highly significant association between abnormalities in the epidermal barrier and risk of developing early-onset, severe and persistent AD.31,35,43 Of note, these may be due to both mutations of genes encoding proteins such as FLG, as well as modulation of epidermal protein levels by Th2 cytokines.44,45 The Th2 cytokines interleukin (IL)-4 and IL-13, which are overexpressed in the acute skin lesions of AD patients, can down-regulate FLG, LOR and IVL, potentially further exacerbating both the epidermal skin barrier defects.44,45 A critical link between the barrier defect in AD patients with FLG mutations and Th2 polarization could be explained in part by enhanced allergen penetration through the damaged epidermis accompanied by increased production of thymic stromal lymphopoietin (TSLP) by keratinocytes leading to a Th2-type milieu.46 Importantly, patients with FLG gene mutations are at an increased risk for developing asthma, but only in the context of having AD, pointing to the importance of allergic sensitization through a damaged skin barrier.44,47,48 Conversely, AD patients with more polarized Th2-type disease with allergies and asthma and increased biomarkers including serum IgE, TSLP and cutaneous T cell-attracting chemokine were also more likely to have severe skin disease complicated by eczema herpeticum (EH), Staphylococcus aureus or molluscum infections.49 In addition, patients with FLG mutations have been found to have an increased risk for EH, a serious complication of AD.46 Using a proteomics approach, Howell et al identified S100/A11 as a target in Th2 cytokine-mediated inhibition of FLG and the antimicrobial peptide, HBD-3, expression in AD skin, pointing to immune dysregulation effecting both epidermal barrier integrity and innate immune response.50 Still, the relationship of skin barrier and immune abnormalities to the increased susceptibility to microbial colonization and infections remains to be fully elucidated.34 Of interest, emerging observations that topical calcineurin inhibitors can in part correct the barrier defect in AD and that gentamicin can restore the production of functional FLG chains provides further evidence of the complex relationship of the epidermal barrier and the immune system.51
It has been shown that skin barrier function as assessed by transepidermal water loss (TEWL) is intrinsically compromised in children with AD, but not in children with other allergic conditions.52 In addition, TEWL was higher in white than in African American children with AD and the magnitude of skin barrier dysfunction correlated with disease severity.52 While TEWL might be a useful biomarker in AD, racial and pigmentation differences will need to be considered. Recently, authors examined TEWL and other parameters of epidermal barrier and showed improvement in all parameters when AD patients were treated with both a topical steroid (betamethazone valerate) and a topical calcineurin inhibitor (pimecrolimus) applied to matched lesions.53 Both treatments normalized epidermal differentiation and reduced epidermal hyperproliferation. Betamethazone valerate was better in reducing clinical symptoms and epidermal proliferation, however it induced epidermal thinning. In contrast, pimecrolimus did not cause skin atrophy. Therefore, we could conclude that pimecrolimus may be used for long-term treatment, and betamethazone valerate might be more useful for treatment of acute exacerbations of AD.54
Figures and Tables
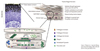
Figure
Filaggrin (FLG) expression and functions in the epidermal skin barrier. FLG is expressed in the granular layer of epidermis. Profilaggrin is dephosphorylated and cleaved by several endoproteases including caspase-14 to generate FLG, and then FLG is degraded into free amino acids (NMF) by caspase-14.
References
1. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009. 124:1251–1258.e23.
2. Bieber T. Atopic dermatitis. Ann Dermatol. 2010. 22:125–137.
3. Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010. 125:4–13.
4. Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994. 30:35–39.
5. Spergel JM. Atopic march: link to upper airways. Curr Opin Allergy Clin Immunol. 2005. 5:17–21.
6. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011. 3:67–73.
7. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006. 155:145–151.
8. Boguniewicz M, Abramovits W, Paller A, Whitaker-Worth DL, Prendergast M, Cheng JW, Wang P, Tong KB. A multiple-domain framework of clinical, economic, and patient-reported outcomes for evaluating benefits of intervention in atopic dermatitis. J Drugs Dermatol. 2007. 6:416–423.
9. Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008. 25:1–6.
10. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, Cardinale I, Lin P, Bergman R, Bowcock AM, Krueger JG. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009. 124:1235–1244.e58.
11. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009. 9:437–446.
12. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008. 121:1337–1343.
13. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005. 6:328–340.
14. Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001. 114:3069–3070.
15. Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009. 124:485–493. 493.e1
16. Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981. 69:271–318.
17. Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D'Herde K, Hachem JP, Borgonie G, Presland RB, Schoonjans L, Libert C, Vandekerckhove J, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007. 9:666–674.
18. Nicotera P, Melino G. Caspase-14 and epidermis maturation. Nat Cell Biol. 2007. 9:621–622.
19. Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986. 115:84–92.
20. Steven AC, Bisher ME, Roop DR, Steinert PM. Biosynthetic pathways of filaggrin and loricrin--two major proteins expressed by terminally differentiated epidermal keratinocytes. J Struct Biol. 1990. 104:150–162.
21. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995. 270:17702–17711.
22. Rice RH, Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977. 11:417–422.
23. O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009. 124:R2–R6.
24. Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011. 127:1110–1118.
25. Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004. 17:Suppl 1. 6–15.
26. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008. 17:1063–1072.
27. Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998. 101:1614–1622.
28. De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011. 127:773–786.e1-7.
29. Jarnik M, de Viragh PA, Schärer E, Bundman D, Simon MN, Roop DR, Steven AC. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J Invest Dermatol. 2002. 118:102–109.
30. Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, Kawachi Y, Suga Y, Zhou Z, Huber M, Hohl D, Kartasova T, Jarnik M, Steven AC, Roop DR. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000. 151:389–400.
31. Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, Irvine AD, Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009. 123:1361–1370.e7.
32. Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Høgh JK, Hellgren LI, Jemec GB, Agner T, Weidinger S. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy. 2010. 65:911–918.
33. Morar N, Willis-Owen SA, Moffatt MF, Cookson WO. The genetics of atopic dermatitis. J Allergy Clin Immunol. 2006. 118:24–34.
34. Leung DY. Our evolving understanding of the functional role of filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009. 124:494–495.
35. Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, Klopp N, Wagenpfeil S, Zhao Y, Liao H, Lee SP, Palmer CN, Jenneck C, Maintz L, Hagemann T, Behrendt H, Ring J, Nothen MM, McLean WH, Novak N. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006. 118:214–219.
36. Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007. 127:1282–1284.
37. O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008. 122:689–693.
38. Stemmler S, Parwez Q, Petrasch-Parwez E, Epplen JT, Hoffjan S. Two common loss-of-function mutations within the filaggrin gene predispose for early onset of atopic dermatitis. J Invest Dermatol. 2007. 127:722–724.
39. Brown SJ, Sandilands A, Zhao Y, Liao H, Relton CL, Meggitt SJ, Trembath RC, Barker JN, Reynolds NJ, Cordell HJ, McLean WH. Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol. 2008. 128:1591–1594.
40. Nomura T, Sandilands A, Akiyama M, Liao H, Evans AT, Sakai K, Ota M, Sugiura H, Yamamoto K, Sato H, Palmer CN, Smith FJ, McLean WH, Shimizu H. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol. 2007. 119:434–440.
41. Zhang H, Guo Y, Wang W, Shi M, Chen X, Yao Z. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy. 2011. 66:420–427.
42. Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, Mukhopadhyay S, Smith GD, Palmer CN, McLean WH, Irvine AD. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008. 121:872–877.e9.
43. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, ORegan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006. 38:441–446.
44. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007. 120:150–155.
45. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008. 126:332–337.
46. Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, Schneider L, Hanifin JM, Gallo RL, Gao L, Beaty TH, Beck LA, Barnes KC, Leung DY. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009. 124:507–513. 513.e1–513.e7.
47. McLean WH, Palmer CN, Henderson J, Kabesch M, Weidinger S, Irvine AD. Filaggrin variants confer susceptibility to asthma. J Allergy Clin Immunol. 2008. 121:1294–1295. author reply 1295-6.
48. Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006. 38:399–400.
49. Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, Paller AS, Lieff S, Reese J, Zaccaro D, Milgrom H, Barnes KC, Leung DY. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009. 124:260–269. 269.e1–269.e7.
50. Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to down-regulate keratinocyte differentiation. J Invest Dermatol. 2008. 128:2248–2258.
51. Jung T, Stingl G. Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008. 122:1074–1081.
52. Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa'ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008. 121:725–730.e2.
53. Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, Schwarz T, Fölster-Holst R, Proksch E. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009. 124:R19–R28.
54. Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, Hamid Q, Kapp A, Leung DY, Lipozencic J, Luger TA, Muraro A, Novak N, Platts-Mills TA, Rosenwasser L, Scheynius A, Simons FE, Spergel J, Turjanmaa K, Wahn U, Weidinger S, Werfel T, Zuberbier T. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/ PRACTALL Consensus Report. J Allergy Clin Immunol. 2006. 118:152–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download