Abstract
Eosinophilia is common feature of many disorders, including allergic diseases. There are many factors that influence the production, migration, survival and death of the eosinophil. Apoptosis is the most common form of physiological cell death and a necessary process to maintain but limit cell numbers in humans and other species. It has been directly demonstrated that eosinophil apoptosis is delayed in allergic inflammatory sites, and that this mechanism contributes to the expansion of eosinophil numbers within tissues. Among the proteins known to influence hematopoiesis and survival, expression of the cytokine interleukin-5 appears to be uniquely important and specific for eosinophils. In contrast, eosinophil death can result from withdrawal of survival factors, but also by activation of pro-apoptotic pathways via death factors. Recent observations suggest a role for cell surface death receptors and mitochondria in facilitating eosinophil apoptosis, although the mechanisms that trigger each of these death pathways remain incompletely delineated. Ultimately, the control of eosinophil apoptosis may someday become another therapeutic strategy for treating allergic diseases and other eosinophil-associated disorders.
In Act 3, scene 1 of Shakespeare's Hamlet, the famous phrase "To be or not to be, that is the question" is uttered. In the context of this review, decisions in the bone marrow "to be or not to be" an eosinophil, and to "survive or not survive" are among the questions that will be addressed. Their role in homeostasis and in inflammation and immunity will also be covered. By necessity, some of the data relevant to these topics will be derived from in vitro studies and animal (mostly murine) studies, but whenever possible, human findings will be highlighted.
Eosinophils are produced in the bone marrow from multipotent hematopoietic stem cells. Hematopoietic differentiation involves the commitment of multipotent progenitors to a given lineage, followed by the maturation of the committed cells. From these stem cells, the myeloid lineage allows the development of the myeloblast with shared properties of basophils and eosinophils, and then into a separate eosinophil lineage.1 Each of the steps that ultimately lead to mature eosinophils is under the fine regulation of soluble mediators and transcription factors.
Several transcription factors are involved in eosinophilic lineage. Forced expression of the transcription C/EBP members (CCAAT/enhancer-binding protein family) in progenitor cells induces myeloid and eosinophil differentiation.2 PU.1, an ETS transcription factor family member, is only expressed in hematopoietic cells. At an early time point of the differentiation, PU.1 is involved in the transition between lymphoid and myeloid lineage. PU.1 expression level determines the fate of the cell and is necessary for dictating monocyte/macrophage and dendritic cell commitment and differentiation, as well as for neutrophil differentiation. In addition, high levels of PU.1 lead to an increase in myeloid differentiation.3 In most cells, PU.1 antagonizes GATA-1 (a zinc finger family member), the latter of which has synergistic activity in regulating eosinophil lineage specification and eosinophil granule protein transcription.4 The interferon consensus sequence binding protein (ICSBP) is also a key transcription factor for eosinophils and is demonstrated by a loss of eosinophils in ICSBP-deficient mice.5 Of these transcription factors, GATA-1 is clearly the most important for eosinophil lineage specification, based on loss of the eosinophil lineage in mice harboring a targeted deletion of the high affinity GATA-binding site in the GATA-1 promoter,6 and based on eosinophil differentiation experiments in vitro.7
Cytokines are indispensable for hematopoietic cell development, differentiation and maturation. Located on chromosome 5q31, IL-3, IL-5, and granulocyte/macrophage-colony stimulating factor (GM-CSF) are cytokines that are particularly important in regulating eosinophil development.8 These "eosinophilopoietins" likely provide permissive proliferative and differentiation signals following the instructive signals specified by the transcription factors GATA-1, PU.1, and C/EBPs. IL-3 and GM-CSF also induce the differentiation of other myeloid cells such as the mast cell, but IL-3, GM-CSF and IL-5 synergize toward the differentiation of eosinophils. Of these three cytokines, IL-5 is the most specific to the eosinophil lineage and is responsible for selective terminal differentiation of eosinophils.9 IL-5 also stimulates the release of eosinophils from the bone marrow into the peripheral circulation.10 The critical role of IL-5 in regulating eosinophils in humans has been demonstrated in several clinical trials with humanized anti-IL-5 antibodies (mepolizumab and reslizumab), currently a non-FDA approved drug, which dramatically lowers eosinophil levels in the blood in part by preventing eosinophil maturation in the bone marrow with arrest at the myelocyte and metamyelocyte stages.11,12
The life cycle of the eosinophil may be divided into bone marrow, blood, and tissue phases. Although the eosinophil is a formed element of the peripheral circulation, it is primarily a tissue-dwelling cell. In humans the tissue eosinophil/blood ratio is about 100:1.14 Furthermore, eosinophils tend to reside in those tissues where the epithelial surfaces are exposed to the external environment (gut); mast cells primarily reside in these tissues as well. Thus, eosinophils are considered merely to "pass through" the circulation en route to the tissues.15
Once the eosinophil has entered the blood, it has a short half-life, ranging from 8 to 18 hours. After circulating in the blood, eosinophils migrate into the tissues, probably by diapedesis at endothelial intercellular junctions4 by a mechanism involving cytokines and adhesion molecules. Under normal conditions, once eosinophils enter the tissues, most do not recirculate. The tissue life span of eosinophils ranges from 2 to 5 days, depending partly on the tissue studied. However, cytokines increase eosinophil survival in vitro to 14 days or longer; thus, they likely also prolong eosinophil survival in vivo.15
Under baseline conditions, most eosinophils traffic into the gastrointestinal tract where they normally reside within the lamina propria of all segments except the esophagus, where there are normally zero eosinophils.16 The gastrointestinal eosinophil is the predominant population of eosinophils. Under baseline conditions, eosinophil levels in the gastrointestinal tract occur independently of lymphocytes and enteric flora, indicating unique regulation compared with other leukocytes.16 Indeed, the recruitment of gastrointestinal eosinophils is regulated by the constitutive expression of eotaxin-1 (CCL11), demonstrated by the marked decrease of this population of eosinophils in eotaxin-1-deficient mice.17 The importance of eotaxin-1 in regulating the baseline level of eosinophils is reinforced by the observation that mice with a targeted deletion of CCR3 (but not eotaxin-2 (CCL24)-deficient mice) also have a deficiency in gastrointestinal eosinophils.17 In addition to trafficking into the gastrointestinal tract, eosinophils home into the thymus, mammary gland, and uterus under homeostatic conditions, also under the regulation of eotaxin-1.18 Of note, trafficking into the uterus is regulated by estrogen, as eosinophil and eotaxin-1 levels cycle along with estrus.19
While IL-5 does not appear to be nearly as important as chemokines for eosinophil recruitment, locally produced IL-5 does have an important function in increasing the survival of eosinophils once they have reached the tissues.
Eosinophil function has primarily been associated with its role in host defense against parasitic infection. Angiostrongyliasis costaricensis, Ascariasis, Hookworm infection, Strongyloidiasis, Trichinosis, Schistosomiasis, Clonorchiasis, and Paragonimiasis usually cause marked eosinophilia (more than 3,000/µL). The level of eosinophilia parallels the magnitude and extent of tissue invasion by helminth larvae or adults.20
Several studies using helminth infection models have evaluated the propensity of eosinophils to: (1) mediate antibody (or complement) dependent cellular toxicity against helminths in vitro,21 (2) degranulate in the local vicinity of damaged parasites in vivo during helminthic infections, and (3) be required for parasite clearance in experimental parasite infected mice that have been depleted of eosinophils by IL-5 neutralization and/or gene targeting.22
Murine studies are particularly problematic because mice are not the natural hosts of many of the experimental parasites; nevertheless, in some primary infection models, a role for IL-5 in protective immunity has been suggested following infection with Strongyloides venezuelensis, Strongyloides ratti, Nippostrongylus brasiliensis, and Heligmosomoides polygyrus.22 These murine in vivo studies need to be interpreted with caution because IL-5 neutralization may have effects on other IL-5 receptorbearing cells (including murine B cells, unlike its biology in humans).9 Other approaches, including the analysis of CCR3- and eotaxin-1-deficient mice, have recently demonstrated a role for eosinophils in the encystment of larvae in Trichinella spiralis and in controlling the Brugia malayi microfilariae, respectively.23 The role of eosinophils in host defense against helminthic parasites in Schistosoma mansoni infection model has been studied in the two eosinophil lineage ablation mouse lines (DdblGATA and PHIL). They found that eosinophil ablation had no effect on worm burden or on egg deposition, indicating that in mice, eosinophils are not necessary for immunity to this organism.24 Thus, although the debate continues, it seems likely that eosinophils participate in the protective immunity against selected
helminths.
Eosinophil granule proteins are known for their ribonuclease activity (such as human eosinophil cationic protein (ECP, RNase 3) and eosinophil-derived neurotoxin (EDN, RNase 2), and at least 11 eosinophil associated ribonucleases (EAR) orthologs in mice) and have been shown to degrade single-stranded RNA-containing viruses.25 Interestingly, it has recently been shown that viruses (parainfluenza virus, respiratory syncytial virus (RSV), or rhinovirus) induce the release of eosinophil peroxidase (EPO) by eosinophils when co-incubated in the presence of antigen-presenting cells and T cells.26 In fact, ECP and EDN are the most divergent coding sequences in the entire human genome (compared with other primates).25 Despite their divergence, they have conserved ribonuclease activity across species, strongly implicating evolutionary pressure to preserve this critical enzymatic activity. Eosinophils may also have a protective role in other infections, especially against RNA viruses, such as RSV and the related natural rodent pathogen, pneumonia virus of mice (PVM), in vivo.25, 27 Eosinophils are recruited and degranulate in lung tissue in response to human RSV (hRSV) infection.28 Experiments performed in vitro and in several different mouse models suggest mechanisms underlying this aspect of eosinophil recruitment,29 and provide evidence consistent with a role for these cells in promoting viral clearance.30 Paradoxically, an in vitro study has shown that eosinophils may be an important reservoir for the HIV-1 virus in vivo.31
Recent investigation has focused on the role of eosinophils in fungal infections. Indeed, eosinophils release their cytotoxic granule proteins into the extracellular milieu and onto the surface of fungal organisms and kill fungi in a contact-dependent manner.32 Eosinophils use their versatile β2-integrin molecule, CD11b, to adhere to a major cell wall component, β-glucan, but eosinophils do not express other common fungal receptors, such as dectin-1 and lactosylceramide. The I-domain of CD11b is distinctively involved in eosinophil interaction with β-glucan. Interestingly, eosinophils do not react with chitin, another fungal cell wall component, even though chitin is a strong Th2 stimulus.32 Fungal protease activity induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils through protease-activated receptors (PAR-2),33 Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils.34 Challenge in the lung with Aspergillus fumigatus or OVA revealed marked induction of murine eotaxin-2 mRNA.35
Eosinophils rapidly release mitochondrial DNA in response to exposure to bacteria, C5a or CCR3 ligands. The traps contain the granule protein ECP and MBP, and display antimicrobial activity.36 In the extracellular space, the mitochondrial DNA and the granule proteins form extracellular structures that bind and kill bacteria both in vitro and under inflammatory conditions in vivo. After cecal ligation and puncture, IL-5-transgenic, but not wild type, mice show intestinal eosinophil infiltration and extracellular DNA deposition in association with protection against microbial sepsis. This data suggests a previously undescribed mechanism of eosinophil-mediated innate immune responses that might be crucial for maintaining the intestinal barrier function after inflammation-associated epithelial cell damage, preventing the host from uncontrolled invasion of bacteria.
Increases of eosinophils in the tissues, blood, and bone marrow are a hallmark of most asthma phenotypes and, in general, elevated numbers correlate with disease severity (although "non-eosinophilic/non-neutrophilic" asthma is characteristic of bacterial, viral, and pollutant triggers).37 This has led to the hypothesis that the eosinophil is a central effector cell responsible for ongoing airway inflammation. Granule proteins, such as MBP, have been found in bronchoalveolar lavage fluid from patients with asthma in sufficient concentrations to induce cytotoxicity of a variety of host tissues including respiratory epithelial cells in vitro.38 MBP has been indirectly involved in airway hyperreactivity (AHR) due to its ability to directly increase smooth muscle reactivity by causing dysfunction of vagal muscarinic M2 receptors.39 In addition to its effect on tissue, MBP can trigger the degranulation of mast cells and basophils that may also be involved in disease pathogenesis.38 Eosinophils generate cysteinyl leukotrienes (cysLTs) that may lead to increased vascular permeability, mucus secretion, and smooth muscle constriction.40 Indeed, inhibitors of cysLTs are effective therapeutic agents for the treatment of allergic airway disease. Thus, the cell has the potential to cause damage to the airway mucosa and associated nerves through the release of granule-associated basic proteins (which damage nerves and epithelial cells), lipid mediators (which cause bronchoconstriction and mucus hypersecretion), and reactive oxygen species (which generally injure mucosal cells).
Blood eosinophils from patients with asthma have a number of phenotypic alterations, particularly in relation to their adhesive properties. Thus airway eosinophils recovered after antigen challenge have enhanced adhesion to VCAM-1 (CD106) and other ligands including albumin, ICAM-1 (CD54), fibrinogen, and vitronectin. These hyperadhesive properties seem to be mediated by up-regulated and activated αMβ2 (CD11b/18).41 Eosinophils in asthmatics also have increased expression of collagen receptors α1β1 and α2β1 integrins.42
More attention is now given to a possible role for the eosinophil in repair and remodeling processes since there is a well-documented association of tissue eosinophilia and eosinophil degranulation with certain fibrotic syndromes. The cell is the source of several fibrogenic and growth factors, including transforming growth factor (TGF)-β, fibroblast growth factor (FGF)-2, vascular endothelial growth factor (VEGF), matrix metalloprotease (MMP)-9, IL-1β, IL-13, and IL-17. Studies in humans using anti-IL-5 antibodies also support a role for eosinophils in events surrounding deposition of certain matrix proteins within the reticular basement membrane.43 When asthmatics were given three infusions of anti-IL-5 (mepolizumab) this produced about a 90% reduction in blood and bronchial lavage eosinophils but only 55% reduction in bronchial mucosal eosinophils,44 even though several matrix proteins were dramatically reduced by anti-IL-5 treatment.45
In order to provide definitive evidence that eosinophils are key cells in airway remodeling, more effective strategies are required to deplete tissue eosinophils.46,47 Even in animal models of asthma there was residual tissue eosinophilia in the airways after anti-IL-5 administration.48 In a mouse model, ablation of eotaxin chemokines prevented antigen induced pulmonary eosinophilia49 and antagonism of CCR3 reduced eosinophil numbers accompanied by a diminution in asthma pathology.50
There is no firm evidence that eosinophils or their products are directly causative in AHR in clinical asthma. The correlation between blood and tissue eosinophils and the degree of AHR is generally weak or nonexistent. While observed in humans, eosinophil degranulation is not always consistent in murine models.51 Elevated levels of blood and/or lung eosinophils are not constitutively associated with lung changes in studies with transgenic mice over-expressing IL-5 (in T cells, lung epithelial cells, or enterocytes).52,53 Neutralization of IL-5 or IL-5 deficient mice, has reduced lung eosinophilia in allergen-challenged lungs,54 but this reduction is not total and does not always correlate with lung function (AHR).55 For example, antigen-induced AHR occurs in allergic IL-5-deficient BALB/c mice but not in IL-5-deficient mice of the C57BL/6 strain.56 A modest effect is seen in human asthma studies using anti-IL-5 antibodies. Patients with mild to moderate asthma were shown to have decreased circulating and sputum eosinophil levels;12 however, no clinical benefit (e.g., improvement in FEV1) was demonstrated. In subsequent studies, however, mepolizumab therapy reduced exacerbations and improved AQLQ scores in patients with refractory eosinophilic asthma,57 It also reduced the number of blood and sputum eosinophils and allowed prednisone sparing in patients who had asthma with sputum eosinophilia despite prednisone treatment.58 Indeed, clinical studies have shown that AHR correlates with mast cell localization near pulmonary nerves, whereas pulmonary eosinophilia relates more strongly with chronic cough.59
In spite of the progress regarding the description of immunological phenomena associated with atopic dermatitis (AD), the pathogenesis of this disease still remains unclear. The presence of eosinophils or their granules in the inflammatory infiltrate of AD has long been established. Eosinophil numbers as well as eosinophil granule protein levels in peripheral blood are elevated in most AD patients and appear to correlate with disease activity. Interestingly, abundant MBP-positive staining in the skin of AD patients occurs, even in the absence of eosinophils.60 These observations indicate a role for eosinophils in the pathogenesis of AD. Furthermore, AD is associated with increased production of Th2 cytokines including IL-5 and IL-4. In AD, IL-5 would specifically act on eosinophils, resulting in accelerated eosinophilopoiesis, cell activation, and delayed apoptosis, and IL-4 would be responsible for the Th2 response and eosinophil specific chemokine production. Therefore, IL-5 is an interesting target for experimental therapy in this inflammatory disorder of the skin. Such studies might result in new insights into the pathogenic role of eosinophils in AD.
While present in multiple tissues, only GI eosinophils are associated with marked eosinophil degranulation.61 In healthy patients or normal mice, eosinophils are present in the lamina propria throughout the GI tract from the stomach to the colon.61 However, eosinophils are not normally found in Peyers patches, or intraepithelial locations.62 The accumulation of eosinophils in the GI tract is a common feature of numerous disorders such as drug reactions, helminth infections, gastroesophageal reflux disease, hypereosinophilic syndrome (HES), eosinophilic gastroenteritis (EGE), allergic colitis and inflammatory bowel disease.62 Primary eosinophil-associated gastrointestinal diseases (EGIDs) such as eosinophilic esophagitis (EE), eosinophilic gastritis (EG), EGE, eosinophilic enteritis, and eosinophilic colitis are defined as disorders that mainly affect the gastrointestinal tract with eosinophil-rich inflammation in the absence of known causes of eosinophilia (e.g., drug reactions, parasitic infections, malignancy). These are hypersensitivity disorders that lie in the middle of a spectrum ranging from anaphylaxis to Celiac disease.62
Interestingly, the intestine of eotaxin-1 deficient mice is almost completely devoid of eosinophils, and similar results were observed in CCR3 deficient mice, which shows a decreased eosinophil level at baseline, in the jejunum.63
While absent in the normal esophagus, eosinophils markedly accumulate in the esophagus of EE patients. A minimum of 15 eosinophils per high power field is now used as pathological criteria for EE.64 Murine models have demonstrated that IL-5 maintains the systemic eosinophil levels needed for esophageal eosinophilia accumulation.16 Substantial evidence is accumulating that human EE is also associated with a Th2 type immune response and local or systemic Th2 cytokine overproduction; IL-5 mRNA expression is induced in the biopsies of EE patients compared to healthy controls,65 and anti-IL-5 may be effective in some but not all patients with EE.66, 67 In human EE, eotaxin-3 (CCL26) expression strongly correlates with eosinophil numbers.68
A large percentage (~10%) of patients suffering from EGID have an immediate family member with EGID.69 Additionally, several lines of evidence support an allergic etiology: (i) about 75% of patients with EGID are atopic;70 (ii) the severity of disease can sometimes be reversed by institution of food elimination diet;70 and (iii) the common finding of mast cell degranulation in tissue specimens.71 Importantly, recent models of EGID support a potential allergic etiology for these disorders.72 Interestingly, despite the common finding of food-specific IgE in patients with EGID, food-induced anaphylactic responses only occur in a small minority of patients.73 Thus, EGIDs have properties that fall between pure IgE-mediated food allergy and cell-mediated hypersensitivity disorders (e.g., celiac disease).73
HES and chronic eosinophilic leukemia (CEL) are related hematological conditions characterized by sustained hypereosinophilia (>1,500 eosinophils/µL). The term CEL is used when there is evidence that the disease is of clonal origin. A subset of patients with HES have a 800 kb interstitial deletion on chromosome 4q12 that results in the fusion of a gene of unknown function, Fip1-like1 (FIP1L1), with the platelet-derived growth factor receptor-a (PDGFRA).74 Dysregulated tyrosine kinase activity by the FIP1L1-PDGFRA fusion gene has been identified as a cause of clonal HES, called FIP1L1-PDGFRA-positive CEL in humans. However, transplantation of FIP1L1-PDGFRA-transduced hematopoietic stem cells/progenitors (HSC/Ps) into mice results in a chronic myelogenous leukemia-like disease, which does not resemble HES. Because a subgroup of patients with HES show T-cell-dependent IL-5 over-expression, whether the expression of the FIP1L1-PDGFRA fusion gene in the presence of transgenic T-cell IL-5 over-expression in mice induces HES-like disease was studied. Mice that received a transplant of CD2-IL-5-transgenic FIP1L1-PDGFRA positive HSC/Ps (IL-5Tg-F/P) developed intense leukocytosis, strikingly high eosinophilia, and eosinophilic infiltration of non-hematopoietic as well as hematopoietic tissues, a phenotype resembling human HES. The disease phenotype was transferable to secondary transplant recipients, suggesting involvement of a short-term repopulating stem cell or an early myeloid progenitor. Induction of significant eosinophilia is, in this model, specific for FIP1L1-PDGFRA since expression of another fusion oncogene, p210-BCR/ABL, in the presence of IL-5 over-expression is characterized by a significantly lower eosinophilia than IL-5Tg-F/P recipients. These results suggest that FIP1L1-PDGFRA fusion gene is not sufficient to induce a HES/CEL-like disease but requires a second event associated with IL-5 overexpression.75 Finally, treatment with mepolizumab can result in corticosteroid-sparing for patients negative for FIP1L1-PDGFRA who have the hypereosinophilic syndrome.76
IL-5 appears to be the most important and specific survival factor for eosinophils, at least within the human system.77 Besides IL-5, other important locally produced survival factors include GM-CSF and, perhaps to a lesser degree, IL-3, tumor necrosis factor-α (TNF-α), interferon-γ, leptin,78 CD40 engagement79 and others.
Both IL-3 and GM-CSF are pluripotent cytokines with activities on other hematopoietic lineages, whereas IL-5 is selective for the eosinophil lineage and plays a crucial role in driving committed eosinophil progenitor-cell proliferation, terminal differentiation, and post-mitotic priming and activation.80
It has been appreciated for some time that IL-3, GM-CSF and IL-5 enhance eosinophil survival when cultured in vitro for weeks, and thus it might be that in vivo eosinophil persistence in the tissues may be prolonged in their presence. From the human IL-5 data and in animal models, the ability of eosinophils to survive and function in the tissue is becoming an important focus. The development and maturation of eosinophils can occur in situ in peripheral sites of inflammation containing preexisting increased tissue eosinophils. Eosinophil progenitors are released into the circulation to reach such tissue sites.81 Eosinophils can release GM-CSF in an autocrine fashion,82 a cytokine which is stored in association with eosinophil granules.83 Other eosinophil-derived and stored cytokines (e.g., IL-4,84 IL-1385) and chemokines (e.g., RANTES (CCL5))48 may further amplify the inflammatory milieu. Thus, the local self-production of such factors by eosinophils may be important in tissue eosinophil reactions beyond IL-5. Local fibroblasts and epithelial cells produce IL-5 and GM-CSF. Eosinophils may enhance their own survival by directly stimulating CD4+ T cells within tissue to produce IL-5. Nasal explants from atopic patients were shown to survive ex vivo using similar mechanisms to promote extramedullary eosinophil maturation and survival.83 These, as well as lung explants of Brown-Norway rats, exhibited rapid (6 hours) accumulation of MBP-positive cells after allergen challenge of the explants in vitro.86 The major signaling pathway of these events is associated with IL-5 receptor ligation leading to phosphorylation of JAK-2 and Lyn kinases, decreased BAX translocation, and ultimately decreased apoptosis.87 Additionally, GM-CSF appears to have a strong role in inhibiting eosinophil apoptosis at the tissue level. Autocrine GM-CSF stimulation of eosinophils bound to fibronectin, via 4 integrins, promoted eosinophil survival for two weeks.82 Eosinophils, when instilled into the trachea of IL-5 knockout mice, not only survive in the absence of IL-5, but in concert with CD4+ T cells, migrate back into lung, and reconstitute the asthma phenotype of wild-type antigen-challenged animals.88 Overall, while IL-5 is essential in the maturation and differentiation of eosinophils in the bone marrow,89 the recruitment to tissues and function within tissues may be IL-5-independent.
Eosinophil apoptosis can be induced in response to specific ligands of the so-called 'death receptors' of the tumor necrosis factor (TNF) family.90 Studies on the role of TNF-α in the induction of eosinophil apoptosis have yielded inconsistent results. Currently available data suggest that induction of apoptosis occurs only under conditions in which nuclear factor-κB is suppressed.91 If this is not the case, TNF-α may even be anti-apoptotic for eosinophils. For instance, TNF-α was shown to activate the p38 mitogen-activated protein (MAP) kinase pathway resulting in delayed eosinophil apoptosis.92 Moreover, the survival effect of TNF-α under in vitro conditions was suggested to be mediated via GM-CSF induction in eosinophils, via activation of both TNF receptors, TNF-RI and TNF-RII.93 Both TNF-α and IL-1 interact synergistically with IL-4 and IL-13 to augment VCAM-1 expression as well as intercellular adhesion molecule-1 (ICAM-1, CD54) expression.94 Recent data suggest that TNF-α prolongs human eosinophil survival by activating both TNF receptor subtypes and NF-κB, but that the mechanism does not involve production of GM-CSF. Interestingly, glucocorticoids completely reversed TNF-α but not IL-5-afforded eosinophil survival.95 This would imply that the mechanism of TNF-α-induced eosinophil survival differs from that of IL-5. Glucocorticoids have been reported to inhibit NF-κB activation.96 In contrast, IL-5 does not cause discernible activation of NF-κB.97 This supports the idea that TNF-α induced eosinophil survival is mediated via activation of NF-κB pathway.
In vitro interferon (IFN)-α, IFN-β and IFN-γ each suppresses colony formation by both multipotential colony-forming units: granulocyte, erythroid, macrophage, and megakaryocyte and day 7 and 14 colony-forming unit granulocyte-macrophage.98 This effect is not dependent on the presence of monocytes, T or B lymphocytes, hence, IFNs appear able to block colony formation by a direct inhibitory effect on colony-forming cells. IFN-α may also act indirectly to reduce eosinophilia through its ability to up-regulate synthesis of IFN-γ,99 a proinflammatory product of Th1 CD4+ lymphocytes that inhibits differentiation of eosinophils from IL-3- and IL-5-stimulated umbilical cord mononuclear cells as well as their tissue migration.100 Both IFN-α and IFN-γ have complex effects on eosinophils. Both IFNs enhanced eosinophil viability in vitro, with IFN-γ being more effective.101 However, in cultures of human umbilical cord mononuclear cells, eosinophil viability decreased markedly after a 1-week culture with IFN-α or with IFN-γ,102 and the number of apoptotic eosinophils increased as well.
Eosinophils have been shown to express the membrane receptor CD40, the ligation of which results in enhanced eosinophil survival as a consequence of autocrine GM-CSF release. Tissue eosinophils resident in nasal polyp tissue have been shown to have a high constitutive expression of CD40.104 The ligand for CD40, CD40L, is expressed by CD4+ T cells that are also present in nasal polyp tissue, which suggests an intriguing potential for a further relationship between eosinophils and T cells. CD40 engagement also enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 (c-IAP2) expression and suggests a role for this mechanism in allergic inflammation.79 CD40 was not expressed on freshly-isolated blood eosinophils, but was seen following culture.
IL-33 potently induces eosinophil adhesion and CD11b expression and enhances eosinophil survival, albeit not as effectively as IL-5. The IL-33-ST2 pathway might be an important regulator of eosinophil biology in the pathogenesis of Th2-biased allergic diseases.105
GM-CSF, IL-3 and IL-5 are cytokines important for eosinophil survival.101 The signaling pathways involved in cytokine-afforded eosinophil survival are complex and partly unknown. The receptors for IL-3 (CD123), IL-5 (CD125) and GM-CSF (CD116) each have unique α-chains but chare a common β-chain (CD131).106 The β-chain is essential for signal transduction and explains the overlapping activities of these cytokines. Cytokine receptors depend on dimerization for their activation, and upon dimerization, multiple tyrosine residues in the β-chain become phosphorylated.106 In general, the signaling events include the phosphorylation of tyrosine kinases, adapter proteins such as Shc and Grb-2, Ras-MAP kinase pathways, and janus kinase (Jak)-signal transducer and the activator of transcription (STAT) pathways (Fig. 2).
The intracellular pathways important in the inhibition of eosinophil apoptosis and their subsequent enhanced survival by IL-3, GM-CSF and IL-5 include the Lyn, Jak2, Raf1 and MAP kinases.108 Indeed, intracellular levels of protein tyrosine phosphorylation appear to be vital in determining whether an eosinophil will exhibit prolonged survival or undergo apoptosis, and a role for both tyrosine phosphorylation and the tyrosine kinase Lyn was recently demonstrated in Fas receptor-mediated apoptosis in eosinophils.109 The picture is complicated further by the observation that levels of intracellular reactive oxygen species in human eosinophils also appear to be involved in regulating their apoptosis and that antioxidants blocked Fasmediated eosinophil death.77
MAP kinases are serine and threonine kinases, which can be activated by phosphorylation in kinase cascades. The members include extracellular-regulated kinase (ERK) 1, 2, 5 and 6, JNK/SAPK, and various isoforms of p38.110
p38 MAP kinase is constitutively activated in surviving eosinophils, and when eosinophils are dying, it is de-activated in those cells. Furthermore inhibition of p38 MAP kinase activity enhances spontaneous apoptosis.92 These results suggest that p38 MAP kinase is involved in the signaling that supports eosinophil survival. The factor(s) that initially lead to p38 activation in eosinophils currently remain unknown.92 This leads to the interesting hypothesis that compounds that inhibit p38 MAP kinase activity may reduce eosinophilia. In fact, this may be true as a p38 MAP kinase inhibitor, SB239063 has been shown to almost completely abolish lung eosinophilia in ovalbumin-sensitized and challenged mice.111 In contrast to p38 MAP kinase, inhibition of ERK 1/2 activity seems not to alter the rate of spontaneous apoptosis in eosinophils.92
Sodium salicylate has been reported to induce JNK and p38 MAP kinase activation in human eosinophils as well as to induce apoptosis, but the effects of sodium salicylate on apoptosis could not be reversed by inhibiting JNK and p38 with antisense oligodeoxynucleotides or with the specific p38 MAP kinase inhibitor SB203580,112 thus suggesting that JNK and p38 MAP kinase are not mediating sodium salicylate-induced eosinophil apoptosis.
Despite a great variety of available apoptotic stimuli, final changes in the dying cell are similar and many of the signaling events appear to converge into common mechanisms involving activation of cysteine-containing proteases that cleave their target proteins at specific aspartic acids (caspases) (Fig. 3). Caspases are part of a family that so far comprise 14 members.113 They are present in the cells as inactive zymogens that must be cleaved to generate free catalytic subunits able to associate and form active heterotetramers.
The presence of caspases 3, 6, 7, 8 and 9 in eosinophils has been described and these caspases are associated with spontaneous eosinophil apoptosis.115 The family of caspases can be divided into two functional subgroups, the initiator and the executioner caspases. Many apoptotic responses are initiated by activation of the initiator caspases-8 or -9. Initiator caspases necessitate special mechanisms of activation of zymogens. For instance, caspase-8 can be activated following recruitment and clustering at multicomponent apoptosis-signaling complexes, resulting from ligation of cell surface molecules of the TNF receptor family, presumably by auto-processing of the zymogens according to the induced-proximity model.116 Caspase-9 is activated by recruitment to Apaf-1 in the presence of ATP following release of cytochrome c from mitochondria.117
Activation of either of these caspases can result in activation of executioner caspases such as caspase-3, leading eventually to apoptosis. Caspase-3 has been shown to be an important effector caspase in eosinophils following mitochondrial activation involving the Bcl-2 family protein Bax.115
IL-5 prevents the caspase activation.115 In one study, a caspase-9 inhibitor blocked eosinophil apoptosis, confirming the view that mitochondria are involved in pro-apoptotic signaling in eosinophils.118 In addition, broad-range caspase inhibitors also blocked eosinophil apoptosis, suggesting that caspases are indeed critical elements of the death machinery in eosinophils.
There have been several reports suggesting the involvement of members of the Bcl-2 family in the regulation of apoptosis that usually regulate the pro-apoptotic activity of mitochondria. This has been somewhat surprising since granulocytes have been described as cells with limited numbers of mitochondria.119 Recently published work, however, suggests that eosinophils contain small numbers of mitochondria, which are involved in the induction of apoptosis.118
Pro-apoptotic Bax molecules have been found to be expressed at high levels in eosinophils.120 Moreover, eosinophil apoptosis was associated with translocation of cytosolic Bax into the outer membrane of mitochondria where it forms pores, allowing the release of pro-apoptotic factors such as cytochrome c.115 Clearly, high levels of Bax may contribute but may not be sufficient to shorten the life span of eosinophils. The more proximal mechanisms that are responsible for Bax translocation to mitochondria in the absence of sufficient stimulation with survival cytokines remain to be determined.
Besides the pro-apoptotic Bax, eosinophils also express anti-apoptotic members of the Bcl-2 family. For instance, Bcl-xL was shown to play an antiapoptotic role and was inducible by IL-5 in eosinophils.120 In contrast, Bcl-2 appears not to be expressed in eosinophils,120 although there are some contrasting reports in the literature.121
Fas (CD95, APO-1) is a cell surface receptor expressed on many cells including eosinophils, which mediates apoptosis when ligated by agonistic antibodies or its natural ligand FasL. It seems that IFN-γ and TNF-α, both alone and synergistically, increase Fas receptor expression whereas IL-3/IL-5/GM-CSF do not modulate constitutive Fas receptor expression.122 After treatment with Fas antibody, electron microscopy of eosinophils and gel electrophoresis of DNA extracted from eosinophils demonstrated changes consistent with apoptosis. These data demonstrate that Fas antigen can modify eosinophil survival by inducing apoptosis through a pathway that is, at least in part, independent of the survival-promoting effects of IL-5.123 Fas-mediated apoptosis in eosinophils can be only partially overcome by IL-5124 and it can be further enhanced by glucocorticoids.125 Cross-linking of Fas can induce eosinophil apoptosis in ex vivo conditions in nasal polyps126 and in vivo in mouse lung.127 Interestingly, blood and tissue eosinophils from some donors did not express functional Fas receptors, although Fas protein was normally expressed in these cells.126
The signaling events induced by Fas in human eosinophils remain largely unknown. Ligation of Fas by agonistic antibody has been shown to result in tyrosine phosphorylation of several intracellular proteins and tyrosine kinase inhibitors lavendustin A and genistein have been reported to block Fas-receptor-induced cell death.109 Lyn phosphorylation by Fas has been reported, and decreases in the expression of Lyn by antisense technique resulted in partial reversal of Fas-induced cell death.109 Similarly, nitric oxide and cyclic adenosine 3':5'-monophosphate (cAMP) and cGMP have been reported to reverse Fas-induced eosinophil death.128 Fas-ligation induces caspase-3 and -8 activation and loss of mitochondrial membrane potential (ΔΨm). Interestingly, incubation of eosinophils with bongkrekic acid, an inhibitor of mitochondrial permeability transition pore opening, failed to modify Fas mediated loss in ΔΨm and apoptosis, whereas caspase inhibitors Z-VAD fmk (broad spectrum), Z-IETD-fmk (caspase-8) and Z-DEVD-fmk (caspase-3) inhibited Fas-induced apoptosis indicating that caspase-3 and -8 might play a role, but loss of ΔΨm is not involved in the signaling by Fas in eosinophils.124
More recently, a selective mechanism for the induction of human eosinophil apoptosis by surface Siglec-8 (sialic acid-binding immunoglobulin-like lectin 8) cross-linking has been identified.112 Siglec-8 is expressed on eosinophils but also on basophils and mast cells. Such cross-linking in eosinophils leads to increased caspase 3 activity, which is an enzyme responsible for the degradation of cellular contents in the final stages of the apoptotic pathway leading to increased cell apoptosis. This is the case even in the presence of IL-5 and GM-CSF.
Mechanistic studies implicated both caspases and reactive oxygen species generation resulting in mitochondrial injury in this cell death. A paradigm has emerged that engagement of Siglec-8 activates the apoptotic pathway involving generation of reactive oxygen species leading to down-stream mitochondrial dysfunction and caspase cleavage before apoptotic death ensues.129
One of the initially confusing observations regarding Siglec-8-induced cell death was that unlike most other eosinophil death pathways that can be overridden by counterbalanced survival signals such as those provided by the cytokines IL-5 and GM-CSF, Siglec-8-induced death was enhanced by these cytokines in that cells would die even more readily with even less of a Siglec-8 engagement signal.112 These results were subsequently confirmed in eosinophils primed in vivo in humans following allergen bronchoprovocation, and primed cells no longer used caspases in the apoptosis process, instead relying exclusively on reactive oxygen species generation and mitochondrial injury.130 Overall, these data suggest that activated eosinophils might be particularly susceptible to pharmacologic approaches that engage Siglec-8.
Intravenous immunoglobulin (IVIG) preparations that are used commercially contain autoantibodies to Siglec-8 at a high enough titer so as to also induce eosinophil apoptosis in vitro, especially in cytokine-primed cells.131
Using IL-5 transgenic mice and Northern blotting, Siglec-E, -F and -G were all expressed at the mRNA level in mouse eosinophils, but patterns of expression were markedly increased in IL-5 transgenic mice only for Siglec-F and Siglec-G.132 Ultimately, it was not until antibodies were generated that this issue was completely resolved, and it is now clear that Siglec-G is expressed on B lymphocytes133 while Siglec-F is most prominently expressed by mouse eosinophils and is considered the closest functional paralog to Siglec-8.134
Systemic administration of two different types of Siglec-F antibodies led to profound depletion of circulating and tissue eosinophils. It appears that the reduction in eosinophil numbers in vivo was due to apoptosis based on ex vivo studies with blood samples from Siglec-F-treated mice, as well as studies in which eosinophils from IL-5 transgenic mice were exposed to Siglec-F antibody in vitro and characteristic changes indicative of apoptosis were seen.135 Administration of anti-Siglec-F antibody in a mouse model of eosinophilic gastroenteritis significantly reduced levels of eosinophilic inflammation in the intestinal mucosa and this was associated with reduced intestinal permeability changes, normalization of intestinal villous crypt height, and restoration of weight gain,136 Siglec-F antibody administration also significantly reduced levels of allergen-induced eosinophilic airway inflammation and features of airway remodeling in a model of chronic mouse model of asthma, especially subepithelial fibrosis, by reducing the number of eosinophils and increasing the number of apoptotic eosinophils in lung and bone marrow.137
TGF-β is a pleiotropic immunoregulatory cytokine that, for instance, antagonizes the effects of IL-5 on eosinophils.138 In addition to blocking the anti-apoptotic effects of IL-5, it also inhibits eosinophil degranulation and cytokine production. The mechanisms of this inhibitory effect of TGF-β are unknown. It has been demonstrated that TGF-β blocks tyrosine phosphorylation of Jak2 and Lyn tyrosine kinases.139 Furthermore, it inhibits the activation of ERK MAP kinase and Stat1 nuclear factor. However, the signaling molecules mediating these effects have not yet been identified. Tyrosine phosphatases have been studied, but do not seem to be involved. It is possible that TGF-β activates some of the newly described inhibitors of tyrosine kinases, which subsequently mediate its inhibitory effects.
CD30, a member of the TNFR family, was originally identified as Ki-1, an antigen expressed on Reed-Sternberg cells in Hodgkin and non-Hodgkin lymphomas, particularly diffuse, large cell lymphoma and immunoblastic lymphoma.140 TNFR family members, including two receptors for TNF, the nerve growth factor receptor, Fas, CD27, CD40, OX40, 4-1BB, TRAIL receptors 1, 2, 3 and 4, as well as several soluble receptors of mammalian and viral origin, participate in cellular activation, induction of survival and/or apoptosis.141
The roles of the nuclear receptor family in eosinophil functions have not yet been clarified. CD30 stimulation recently has been shown to cause eosinophil-specific apoptosis.142 It has been further reported that CD30 stimulation markedly induced Nur77 and NOR1, NR4A nuclear receptor family, during eosinophil-specific apoptosis.143 However, CD30 stimulation did not alter Nurr1 expression in this system. Fas-signaling has also been shown to induce eosinophil apoptosis, but this effect is not eosinophil-specific; nor were Nur77 or NOR1 induced before apoptosis under these circumstances. Gene expression of the NR4A nuclear receptor family and subsequent eosinophil apoptosis were down-regulated by a MAP kinase inhibitor, but not by a p38 inhibitor. ERK1/2 phosphorylation by MEK1/2 downstream of CD30 signaling and upstream of the nuclear receptor family may be involved in apoptosis, but there still is no information on events downstream of these nuclear receptors.
Glucocorticoids can cause a striking reduction in eosinophil numbers in vivo144 and in their inhaled form remain the mainstay of anti-inflammatory therapy in asthma.145 Although the precise mechanism of action of steroids remains to be determined, glucocorticoids are likely to exert their effects on eosinophils by accelerating their apoptosis and engulfment by lung macrophages,146 by inhibiting the production of survival-enhancing cytokines or both. It is now clear that glucocorticoids suppress the transcription of the IL-5 and other cytokine genes. This inhibition of transcription is the consequence of inhibition of the potent inflammatory transcription factor NF-κB (Fig. 4).147
Eosinophils are highly sensitive to apoptosis induction by corticosteroids that can only be overcome by high concentrations of IL-5.148 Previous work with eosinophils derived from both healthy and asymptomatic allergic individuals has demonstrated an involvement of caspase-3 and -8 in glucocorticoid-induced apoptosis.149 One recent study has demonstrated that in vitro corticosteroid treatment of eosinophils in nasal polyp tissue sections enhanced their apoptosis induction,150 suggesting that eosinophil apoptosis induction by glucocorticoids might be relevant to their anti-inflammatory effects in sinus disease. Interestingly, there is evidence that the bronchodilating β-adrenoreceptor agonists block the anti-apoptotic effects of corticosteroids on eosinophils,151 which might have important implications for the over-usage of β2-agonists in asthma therapy.
Lidocaine and its analogues have been reported to inhibit IL-5-mediated eosinophil survival. This inhibition cannot be overcome by increasing concentrations of IL-5 and is not due to the blocking of Na+ channels by lidocaine.152
IRp60/CD300a is an inhibitory receptor on eosinophil. Although CD33 and p75/AIRM could inhibit proliferation of myeloid cell precursors, IRp60/CD300a could not suppress this feature, which suggests distinct functions for various inhibitory receptors.153 CD300a/IRp60 can also inhibit eosinophil survival.154 In contrast to Siglec-8, which induces eosinophil apoptosis, IRp60/CD300a inhibits survival signals delivered to eosinophils via the IL-3/IL-5/GM-CSF receptor βc.154 Cross-linking experiments have revealed that upon IRp60/CD300a activation, JAK2, p38, and extracellular signal-regulated kinase 1/2 phosphorylation are inhibited, probably from the recruitment of SHP-1 and not SHP-2.
The different outcome of Siglec-8 activation (induction of apoptosis) as opposed to IRp60/CD300 activation (inhibition of survival signals) may be partially explained by the fact that Siglec-8 contains both ITIM and ITSM motifs. ITSM motifs may recruit either inhibitory phosphatases such as SHP-1 and/or SHP-2 or activatory molecules such as slam-associated protein (SAP) and/or 2-Ewing's sarcoma-FLI activated transcript 2 (EAT-2).155
Based on extensive research related to eosinophil biology, especially in mice and humans, have revealed much about the mechanisms involved in the birth, migration, accumulation, participation and ultimately the death of this cell. Acceleration of eosinophil apoptosis can be achieved by elimination of eosinophil survival factors, and by promotion of death signals. These are no being exploited using new pharmacologic agents, including biologicals, in animals and humans, and in the process of testing these drugs we are learning even more about the role of the eosinophil in a variety of disorders. Ultimately, the control of eosinophils may someday become another therapeutic strategy in the treatment of asthma, HES, gastrointestinal eosinophilic diseases and other related eosinophil disorders.
Figures and Tables
Fig. 2
The intracellular signalling pathways of survival-prolonging cytokines IL-3, IL-5 and GM-CSF.107
ACKNOWLEDGMENTS
This work was supported in part by grant AI072265 from the National Institutes of Health. Dr. Bochner also received support for Human Immunology Research from the Dana Foundation and as a Cosner Scholar in Translational Research from the Johns Hopkins University.
References
1. Boyce JA, Friend D, Matsumoto R, Austen KF, Owen WF. Differentiation in vitro of hybrid eosinophil/basophil granulocytes: autocrine function of an eosinophil developmental intermediate. J Exp Med. 1995. 182:49–57.
2. Nerlov C, McNagny KM, Doderlein G, Kowenz-Leutz E, Graf T. Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev. 1998. 12:2413–2423.
3. McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002. 195:F43–F47.
4. Du J, Stankiewicz MJ, Liu Y, Xi Q, Schmitz JE, Lekstrom-Himes JA, Ackerman SJ. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem. 2002. 277:43481–43494.
5. Milanovic M, Terszowski G, Struck D, Liesenfeld O, Carstanjen D. IFN consensus sequence binding protein (Icsbp) is critical for eosinophil development. J Immunol. 2008. 181:5045–5053.
6. Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002. 195:1387–1395.
7. Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, Kato Y, Onodera M, Minegishi N, Yamamoto M, Fukao K, Taniguchi H, Nakauchi H, Iwama A. Essential and instructive roles of GATA factors in eosinophil development. J Exp Med. 2002. 195:1379–1386.
8. Lopez AF, Begley CG, Williamson DJ, Warren DJ, Vadas MA, Sanderson CJ. Murine eosinophil differentiation factor. An eosinophilspecific colony-stimulating factor with activity for human cells. J Exp Med. 1986. 163:1085–1099.
9. Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992. 79:3101–3109.
10. Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin toinduce eosinophil accumulation in vivo. J Exp Med. 1995. 182:1169–1174.
11. Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003. 111:714–719.
12. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000. 356:2144–2148.
13. Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009. 101:81–121.
14. Kita H, Adolphson CR, Gleich GJ, editors. Biology of eosinophils. 1998. 5 ed. St Louis: Mosby.
15. Sur S, Adolphson CR, Gleich GJ, editors. Eosinophils: biochemical and cellular aspects. 1993. 4 ed. St Louis: Mosby.
16. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999. 103:1719–1727.
17. Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimentalallergic lung inflammation. J Biol Chem. 2005. 280:13952–13961.
18. Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils in health and disease. Adv Immunol. 2001. 78:291–328.
19. Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001. 142:4515–4521.
20. Wilson ME, Weller PF, editors. Eosinophilia. 1999. New York: Churchill Livingstone.
21. Butterworth AE. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977. 77:127–168.
22. Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000. 16:202–209.
23. Simons JE, Rothenberg ME, Lawrence RA. Eotaxin-1-regulated eosinophils have a critical role in innate immunity against experimental Brugia malayi infection. Eur J Immunol. 2005. 35:189–197.
24. Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006. 108:2420–2427.
25. Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001. 70:691–698.
26. Davoine F, Cao M, Wu Y, Ajamian F, Ilarraza R, Kokaji AI, Moqbel R, Adamko DJ. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008. 122:69–77. e1–e2.
27. Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999. 190:1465–1478.
28. Kim CK, Koh JY, Han TH, Kim do K, Kim BI, Koh YY. Increased levels of BAL cysteinyl leukotrienesinacute [corrected] RSV bronchiolitis. Acta Paediatr. 2006. 95:479–485.
29. Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005. 18:541–555.
30. Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007. 110:1578–1586.
31. Freedman AR, Gibson FM, Fleming SC, Spry CJ, Griffin GE. Human immunodeficiency virus infection of eosinophils in human bone marrow cultures. J Exp Med. 1991. 174:1661–1664.
32. Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008. 181:2907–2915.
33. Matsuwaki Y, Wada K, White TA, Benson LM, Charlesworth MC, Checkel JL, Inoue Y, Hotta K, Ponikau JU, Lawrence CB, Kita H. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009. 183:6708–6716.
34. Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005. 175:5439–5447.
35. Zimmermann N, Hogan SP, Mishra A, Brandt EB, Bodette TR, Pope SM, Finkelman FD, Rothenberg ME. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J Immunol. 2000. 165:5839–5846.
36. Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008. 14:949–953.
37. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002. 57:643–648.
38. Rothenberg ME. Eosinophilia. N Engl J Med. 1998. 338:1592–1600.
39. Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993. 91:1314–1318.
40. Bandeira-Melo C, Woods LJ, Phoofolo M, Weller PF. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002. 196:841–850.
41. Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006. 35:378–386.
42. Bazan-Socha S, Bukiej A, Pulka G, Marcinkiewicz C, Musial J. Increasedexpression of collagen receptors: alpha1beta1 and alpha-2beta1 integrins on blood eosinophils in bronchial asthma. Clin Exp Allergy. 2006. 36:1184–1191.
43. Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004. 25:477–482.
44. Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003. 167:199–204.
45. Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003. 112:1029–1036.
46. Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004. 305:1773–1776.
47. Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004. 305:1776–1779.
48. Foster PS, Mould AW, Yang M, Mackenzie J, Mattes J, Hogan SP, Mahalingam S, McKenzie AN, Rothenberg ME, Young IG, Matthaei KI, Webb DC. Elemental signals regulating eosinophil accumulation in the lung. Immunol Rev. 2001. 179:173–181.
49. Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005. 175:5341–5350.
50. Wegmann M, Goggel R, Sel S, Erb KJ, Kalkbrenner F, Renz H, Garn H. Effects of a low-molecular-weight CCR-3 antagonist on chronic experimental asthma. Am J Respir Cell Mol Biol. 2007. 36:61–67.
51. Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000. 165:5509–5517.
52. Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990. 172:1425–1431.
53. Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, FosterPS , Rothenberg ME. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002. 277:4406–4412.
54. Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia-the key to asthma? Immunol Rev. 2001. 179:182–191.
55. Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996. 183:109–117.
56. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, andlung damage in a mouse asthma model. J Exp Med. 1996. 183:195–201.
57. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009. 360:973–984.
58. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009. 360:985–993.
59. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002. 346:1699–1705.
60. Davis MD, Plager DA, George TJ, Weiss EA, Gleich GJ, Leiferman KM. Interactions of eosinophil granule proteins with skin: limits of detection, persistence, and vasopermeabilization. J Allergy Clin Immunol. 2003. 112:988–994.
61. Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, McGovern TW, Gleich GJ. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998. 252:418–425.
62. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004. 113:11–28. quiz 9.
63. Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, Wert SE, Rothenberg ME. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998. 95:6273–6278.
64. Gonsalves N, Policarpio-Nicolas M, Zhang Q, Rao MS, Hirano I. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006. 64:313–319.
65. Straumann A, Kristl J, Conus S, Vassina E, Spichtin HP, Beglinger C, Simon HU. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005. 11:720–726.
66. Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006. 118:1312–1319.
67. Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, Beglinger C, Smith DA, Patel J, Byrne M, Simon HU. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010. 59:21–30.
68. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006. 116:536–547.
69. Guajardo JR, Plotnick LM, Fende JM, Collins MH, Putnam PE, Rothenberg ME. Eosinophil-associated gastrointestinal disorders: a world-wide-web based registry. J Pediatr. 2002. 141:576–581.
70. Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002. 109:363–368.
71. Bischoff SC. Mucosal allergy: role of mast cells and eosinophil granulocytes in the gut. Baillieres Clin Gastroenterol. 1996. 10:443–459.
72. Rothenberg ME, Mishra A, Brandt EB, Hogan SP. Gastrointestinal eosinophils. Immunol Rev. 2001. 179:139–155.
73. Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999. 103:717–728.
74. Cools J, Stover EH, Wlodarska I, Marynen P, Gilliland DG. The FIP1L1-PDGFRalpha kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia. Curr Opin Hematol. 2004. 11:51–57.
75. Yamada Y, Rothenberg ME, Lee AW, Akei HS, Brandt EB, Williams DA, Cancelas JA. The FIP1L1-PDGFRA fusion gene cooperates with IL-5 to induce murine hypereosinophilic syndrome (HES)/chronic eosinophilic leukemia (CEL)-like disease. Blood. 2006. 107:4071–4079.
76. Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, Schwartz LB, Rosenwasser LJ, Ring J, Griffin EF, Haig AE, Frewer PI, Parkin JM, Gleich GJ. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008. 358:1215–1228.
77. Bagley CJ, Lopez AF, Vadas MA. New frontiers for IL-5. J Allergy Clin Immunol. 1997. 99:725–728.
78. Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005. 116:1228–1234.
79. Bureau F, Seumois G, Jaspar F, Vanderplasschen A, Detry B, Pastoret PP, Louis R, Lekeux P. CD40 engagement enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 expression: Possible involvement in allergic inflammation. J Allergy Clin Immunol. 2002. 110:443–449.
80. Sanderson CJ. Eosinophil differentiation factor (interleukin-5). Immunol Ser. 1990. 49:231–256.
81. Denburg JA. Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammation. Immunol Today. 1999. 20:111–113.
82. Anwar AR, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion tofibronectin prolongs eosinophil survival. J Exp Med. 1993. 177:839–843.
83. Levi-Schaffer F, Lacy P, Severs NJ, Newman TM, North J, GompertsB , Kay AB, Moqbel R. Association of granulocyte-macrophage colony-stimulating factor with the crystalloid granules of human eosinophils. Blood. 1995. 85:2579–2586.
84. Moqbel R, Ying S, Barkans J, Newman TM, Kimmitt P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan CJ, Durham SR, Kay AB. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995. 155:4939–4947.
85. Schmid-Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G, Blaser K, Wuthrich B, Simon HU. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002. 169:1021–1027.
86. Eidelman DH, Minshall E, Dandurand RJ, Schotman E, Song YL, Yasruel Z, Moqbel R, Hamid Q. Evidence for major basic protein immunoreactivity and interleukin 5 gene activation during the late phase response in explanted airways. Am J Respir Cell Mol Biol. 1996. 15:582–589.
87. Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998. 188:421–429.
88. Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O'Neill KR, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003. 170:3296–3305.
89. Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O'Byrne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000. 106:S242–S246.
90. Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994. 76:959–962.
91. Fujihara S, Ward C, Dransfield I, Hay RT, Uings IJ, Hayes B, Farrow SN, Haslett C, Rossi AG. Inhibition of nuclear factor-kappaB activation un-masks the ability of TNF-alpha to induce human eosinophil apoptosis. Eur J Immunol. 2002. 32:457–466.
92. Kankaanranta H, De Souza PM, Barnes PJ, Salmon M, Giembycz MA, Lindsay MA. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils. J Pharmacol Exp Ther. 1999. 290:621–628.
93. Levi-Schaffer F, Temkin V, Malamud V, Feld S, Zilberman Y. Mast cells enhance eosinophil survival in vitro: role of TNF-alpha and granulocyte-macrophage colony-stimulating factor. J Immunol. 1998. 160:5554–5562.
94. Bochner BS, editor. Cellular adhesion in inflammation. 2003. 6 ed. St. Louis (MO): Mosby.
95. Zhang X, Moilanen E, Adcock IM, Lindsay MA, Kankaanranta H. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002. 71:1523–1534.
96. Wilson SJ, Wallin A, Della-Cioppa G, Sandstrom T, Holgate ST. Effects of budesonide and formoterol on NF-kappaB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med. 2001. 164:1047–1052.
97. Wong CK, Zhang J, Ip WK, Lam CW. Intracellular signal transduction in eosinophils and its clinical significance. Immunopharmacol Immunotoxicol. 2002. 24:165–186.
98. Broxmeyer HE, Lu L, Platzer E, Feit C, Juliano L, Rubin BY. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983. 131:1300–1305.
99. Schandene L, Cogan E, Crusiaux A, Goldman M. Interferon-alpha upregulates both interleukin-10 and interferon-gamma production by human CD4+ T cells. Blood. 1997. 89:1110–1111.
100. Ochiai K, Iwamoto I, Takahashi H, Yoshida S, Tomioka H. Effect of IL-4 and interferon-gamma (IFN-gamma) on IL-3- and IL-5-induced eosinophil differentiation from human cord blood mononuclear cells. Clin Exp Immunol. 1995. 99:124–128.
101. Valerius T, Repp R, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines. A novel class of eosinophil activators with delayed onset of action. J Immunol. 1990. 145:2950–2958.
102. Morita M, Lamkhioued B, Soussi Gounni A, Aldebert D, Delaporte E, Capron A, Capron M. Induction by interferons of human eosinophilapoptosis and regulation by interleukin-3, granulocyte/macrophage-colony stimulating factor and interleukin-5. Eur Cytokine Netw. 1996. 7:725–732.
103. Najib S, Sanchez-Margalet V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002. 220:143–149.
104. Ohkawara Y, Lim KG, Xing Z, Glibetic M, Nakano K, Dolovich J, Croitoru K, Weller PF, Jordana M. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996. 97:1761–1766.
105. Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, NagaseH , Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008. 88:1245–1253.
106. Geijsen N, Koenderman L, Coffer PJ. Specificity in cytokine signal transduction: lessons learned from the IL-3/IL-5/GM-CSF receptor family. Cytokine Growth Factor Rev. 2001. 12:19–25.
107. Kankaanranta H, Moilanen E, Zhang X. Pharmacological regulation of human eosinophil apoptosis. Curr Drug Targets Inflamm Allergy. 2005. 4:433–445.
108. Simon H, Alam R. Regulation of eosinophil apoptosis: transduction of survival and death signals. Int Arch Allergy Immunol. 1999. 118:7–14.
109. Simon HU, Yousefi S, Dibbert B, Hebestreit H, Weber M, Branch DR, Blaser K, Levi-Schaffer F, Anderson GP. Role for tyrosine phosphorylation and Lyn tyrosine kinase in fas receptor-mediated apoptosis in eosinophils. Blood. 1998. 92:547–557.
110. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002. 20:55–72.
111. Underwood DC, Osborn RR, Kotzer CJ, Adams JL, Lee JC, Webb EF, Carpenter DC, Bochnowicz S, Thomas HC, Hay DW, Griswold DE. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther. 2000. 293:281–288.
112. Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003. 101:5014–5020.
113. Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998. 281:1312–1316.
114. Simon HU. Eosinophil apoptosis--pathophysiologic and therapeutic implications. Allergy. 2000. 55:910–915.
115. Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001. 98:2239–2247.
116. Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999. 96:10964–10967.
117. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.
118. Peachman KK, Lyles DS, Bass DA. Mitochondria in eosinophils: functional role in apoptosis but not respiration. Proc Natl Acad Sci U S A. 2001. 98:1717–1722.
119. Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971. 134:907–934.
120. Dibbert B, Daigle I, Braun D, Schranz C, Weber M, Blaser K, Zangemeister-Wittke U, Akbar AN, Simon HU. Role for Bcl-xL in delayedeosinophil apoptosis mediated by granulocyte-macrophage colony-stimulating factor and interleukin-5. Blood. 1998. 92:778–783.
121. El-Gamal Y, Heshmat N, Mahran M, El-Gabbas Z. Expression of the apoptosis inhibitor Bcl-2 in sputum eosinophils from children with acute asthma. Clin Exp Allergy. 2004. 34:1701–1706.
122. Luttmann W, Opfer A, Dauer E, Foerster M, Matthys H, Eibel H, Schulze-Osthoff K, Kroegel C, Virchow JC. Differential regulation of CD95 (Fas/APO-1) expression in human blood eosinophils. Eur J Immunol. 1998. 28:2057–2065.
123. Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood. 1995. 86:1437–1443.
124. Letuve S, Druilhe A, Grandsaigne M, Aubier M, Pretolani M. Involvement of caspases and of mitochondria in Fas ligation-induced eosinophil apoptosis: modulation by interleukin-5 and interferon-gamma. J Leukoc Biol. 2001. 70:767–775.
125. Druilhe A, Cai Z, Haile S, Chouaib S, Pretolani M. Fas-mediated apoptosis in cultured human eosinophils. Blood. 1996. 87:2822–2830.
126. Hebestreit H, Yousefi S, Balatti I, Weber M, Crameri R, Simon D, Hartung K, Schapowal A, Blaser K, Simon HU. Expression and function of the Fas receptor on human blood and tissue eosinophils. Eur J Immunol. 1996. 26:1775–1780.
127. Yamashita K, Takahashi A, Kobayashi S, Hirata H, Mesner PW Jr, Kaufmann SH, Yonehara S, Yamamoto K, Uchiyama T, Sasada M. Caspases mediate tumor necrosis factor-alpha-induced neutrophil apoptosis and downregulation of reactive oxygen production. Blood. 1999. 93:674–685.
128. Hebestreit H, Dibbert B, Balatti I, Braun D, Schapowal A, Blaser K, Simon HU. Disruption of fas receptor signaling by nitric oxide in eosinophils. J Exp Med. 1998. 187:415–425.
129. Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005. 336:918–924.
130. Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008. 38:121–124.
131. von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007. 119:1005–1011.
132. Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevantto mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G). Genomics. 2003. 82:521–530.
133. Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007. 8:695–704.
134. Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005. 15:1125–1135.
135. Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008. 63:1156–1163.
136. Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009. 131:157–169.
137. Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009. 183:5333–5341.
138. Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994. 179:1041–1045.
139. Pazdrak K, Justement L, Alam R. Mechanism of inhibition of eosinophil activation by transforming growth factor-beta. Inhibition of Lyn, MAP, Jak2 kinases and STAT1 nuclear factor. J Immunol. 1995. 155:4454–4458.
140. Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992. 68:421–427.
141. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003. 3:609–620.
142. Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004. 172:2186–2193.
143. Kagaya S, Hashida R, Ohkura N, Tsukada T, Sugita Y, Terakawa M, Tsujimoto G, Katsunuma T, Akasawa A, Matsumoto K, Saito H. NR4A orphan nuclear receptor family in peripheral blood eosinophils from patients with atopic dermatitis and apoptotic eosinophils in vitro. Int Arch Allergy Immunol. 2005. 137:Suppl 1. 35–44.
144. Schleimer RP. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis. 1990. 141:S59–S69.
145. Barnes PJ. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol. 1998. 102:531–538.
146. Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996. 156:4422–4428.
147. Schwiebert LM, Beck LA, Stellato C, Bickel CA, Bochner BS, Schleimer RP. Glucocorticosteroid inhibition of cytokine production: relevanceto antiallergic actions. J Allergy Clin Immunol. 1996. 97:143–152.
148. Walsh GM, Wardlaw AJ. Dexamethasone inhibits prolonged survival and autocrine granulocyte-macrophage colony-stimulating factor production by human eosinophils cultured on laminin or tissue fibronectin. J Allergy Clin Immunol. 1997. 100:208–215.
149. Zangrilli J, Robertson N, Shetty A, Wu J, Hastie A, Fish JE, Litwack G, Peters SP. Effect of IL-5, glucocorticoid, and Fas ligation on Bcl-2 homologue expression and caspase activation in circulating human eosinophils. Clin Exp Immunol. 2000. 120:12–21.
150. Saunders MW, Wheatley AH, George SJ, Lai T, Birchall MA. Do corticosteroids induce apoptosis in nasal polyp inflammatory cells? In vivo and in vitro studies. Laryngoscope. 1999. 109:785–790.
151. Nielson CP, Hadjokas NE. Beta-adrenoceptor agonists block corticosteroid inhibition in eosinophils. Am J Respir Crit Care Med. 1998. 157:184–191.
152. Okada S, Hagan JB, Kato M, Bankers-Fulbright JL, Hunt LW, Gleich GJ, Kita H. Lidocaine and its analogues inhibit IL-5-mediated survival and activation of human eosinophils. J Immunol. 1998. 160:4010–4017.
153. Mingari MC, Vitale C, Romagnani C, Falco M, Moretta L. p75/AIRM1 and CD33, two sialoadhesin receptors that regulate the proliferation or the survival of normal and leukemic myeloid cells. Immunol Rev. 2001. 181:260–268.
154. Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006. 107:1996–2003.
155. Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005. 5:363–374.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




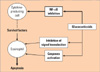
 XML Download
XML Download