Abstract
Canine adenovirus type 2 (CAV-2) is the cause of a major respiratory illness in dogs. In this study, we analyzed adenovirus infections in dogs using 2000~2017 data from the Animal and Plant Quarantine Agency (APQA) and conducted a serological survey of CAV-2 infection in six animal species in Korea. In total, 38 of the 3,179 dog samples were confirmed as canine adenovirus infections. In serological survey, 1,028 dog sera, 160 raccoon dog sera, 100 cattle sera, 257 sow sera, 206 horse sera, and 106 cat sera, collected from January 2016 to July 2018, were screened for the presence of anti-CAV-2 antibodies by virus neutralization test. The seropositivity rates for dogs, raccoon dogs, cattle, sows, horses, and cats were 88.5% (910/1,028), 51.3% (82/160), 85.0% (85/100), 48.6% (125/257), 35.0% (72/206), and 2.8%(3/106), respectively. Among dogs and raccoon dogs, 1.9% (20/1,028) and 8.8%(14/160), respectively, had a virus-neutralizing antibody (VNA) titer of over 1:256. A high CAV-2 VNA titer indicates a repeated vaccination or natural infection in Korean dogs and circulation of CAV-2 in raccoon dog populations.
REFERENCES
1). Davison AJ, Benko M, Harrach. Genetic content and evolution of adenoviruses. J Gen Virol. 2003; 84:2895–908.
2). Linné T. Differences in the E3 regions of the canine adenovirus type 1 and type 2. Virus Res. 1992; 23:119–33.

3). Tham KM, Horner GW, Hunter R. Isolation and identification of canine adenovirus type -2 from the upper respiratory tract of a dog. N Z Vet J. 1998; 46:102–5.
4). Shenk T. Adenoviridae: the virues and their replication. Fields Virology. 4th ed. Vol. 2:p. 2265–2300. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins;2001.
5). Robinson CM, Singh G, Lee JY, Dehghan S, Rajaiya J, Liu EB, et al. Molecular evolution of human adenoviruses. Sci Rep. 2013; 3:1812.

6). Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005; 12:384–93.

7). Gür S, Acar A. A retrospective investigation of canine adenovirus (CAV) infection in adult dogs in Turkey. J S Afr Vet Assoc. 2009; 80:84–6.
8). Whetstone CA. Monoclonal antibodies to canine adenoviruses 1 and 2 that are type-specific by virus neutralization and immunofluorescence. Vet Microbiol. 1988; 16:1–8.

9). Noon KF, Rogul M, Binn LN, Keefe TJ, Marchwicki RH, Thomas R, et al. An enzyme-linked immunosorbent assay for the detection of canine antibodies to canine adenoviruses. Lab Anim Sci. 1979; 29:603–9.
10). Takamura K, Ajiki M, Hiramatsu K, Takemitsu S, Nakai M, Sasaki N. Isolation and properties of adenovirus from canine respiratory tract. Nihon Julgaku Zasshi. 1982; 44:355–7.

11). Choi JW, Lee HK, Kim SH, Kim YH, Lee KK, Lee MH, et al. Canine adenovirus type 1 in a fennec fox (Vulpes zerda). J Zoo Wildl Med. 2014; 45:947–50.

12). Park NY, Lee MC, Kurkure NV, Cho HS. Canine adenovirus type 1 infection of a Eurasian river otter (Lutra lutra). Vet Pathol. 2007; 44:536–9.

13). Yoon SS, Byun JW, Park YI, Kim MJ, Bae YC, Song JY. Comparison of the diagnostic methods on the canine adenovirus type 2 infection. Basic and Applied Pathology. 2010; 2:52–6.

14). Reed LJ, Muench H. A Simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938; 27:493–7.
15). Cabasso VJ, Stebbins MR, Norton TW, Cox HR. Propagation of infectious canine hepatitis virus in tissue culture. Proc Soc Exp Biol Med. 1954; 85:239–45.
16). Benetka V, Weissenböck H, Kudielka I, Pallan C, Rothmüller G, Möstl K. Canine adenovirus type 2 infection in four puppies with neurological signs. Vet Rec. 2006; 158:91–4.
17). Pizzurro F, Marcacci M, Zaccaria G, Orsini M, Cito F, Rosamilia A, et al. Genome sequence of canine adenovirus type 1 isolated from a wolf (Canis lupus) in Southern Italy. Genome Announc. 2017; 20:5.
18). Walker D, Abbondati E, Cox AL, Mitchell GB, Pizzi R, Sharp CP, et al. Infectious canine hepatitis in red foxes (Vulpes vulpes) in wildlife rescue centers in the UK. Vet Rec. 2016; 178:421.
19). Lavan R, Knesl O. Prevalence of canine infectious respiratory pathogens in asymptomatic dogs presented at US animal shelters. J Small Anim Pract. 2015; 56:572–6.

20). Bass EP, Gill MA, Beckenhauer WH. Evaluation of a canine adenovirus type 2 strain as a replacement for infectious canine hepatitis vaccine. J Am Vet Med Assoc. 1980; 177:234–42.
21). Mitchell SA, Zwijnenberg RJ, Huang J, Hodge A, Day MJ. Duration of serological response to canine parvovirus-type 2, canine distemper virus, canine adenovirus type 1 and canine parainfluenza virus in client-owned dogs in Australia. Aust Vet J. 2012; 90:468–73.

22). Bulut O, Yapici O, Avci O, Simsek A, Atli K, Dik I, et al. The serological and virological investigation of canine adenovirus infection on the dogs. Scientific World Journal. 2013; 24:587024.

23). Aoki E, Soma T, Yokoyama M, Matsubayashi M, Sasai K. Surveillance for antibodies against six canine viruses in wild raccoons (Procyon lotor) in Japan. J Wildl Dis. 2017; 53:761–8.

Figure 1.
Number of canine adenovirus (CAV) infections confirmed by the Animal and Plant Quarantine Agency (APQA) since 2000 (Y axis on the left) and number of dog samples sent to the APQA for diagnosis (Y axis on the right). Of 8,179 dog samples, 38 had CAV infections based on the autopsy, pathological and histological findings and polymerase chain reaction.
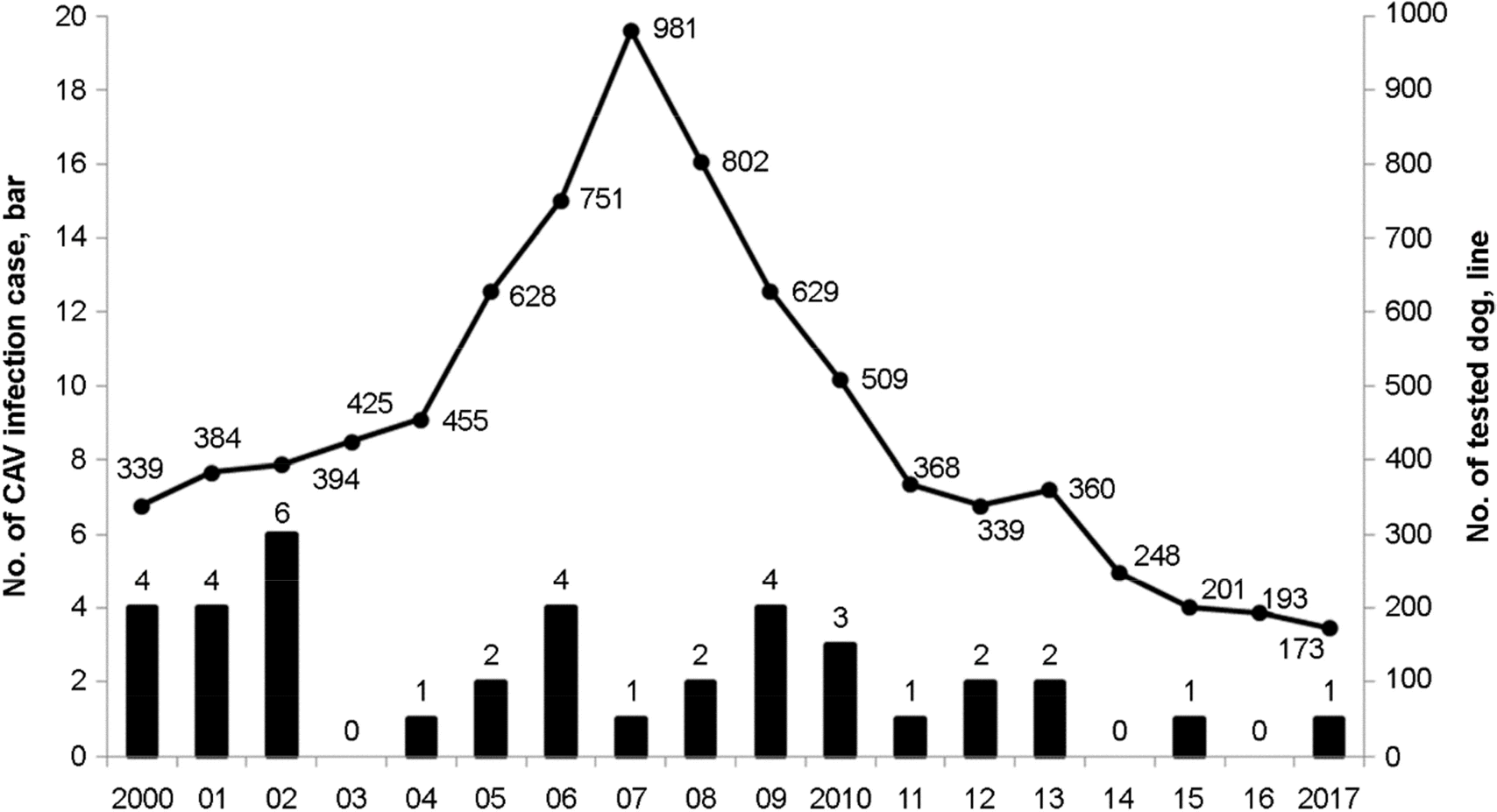
Figure 2.
Sero-prevalence of CAV-2 in dogs, raccoon dogs, cattle, sows, horses, and cats. The overall sero-prevalence of CAV-2 was 88.5% (910/1,028) in dogs, 51.3% (82/160) in raccoon dogs, 85.0% (85/100) in cattle, 48.6% (125/257) in sows, 35.0%(72/206) in horses, and 2.8% (3/106) in cats.
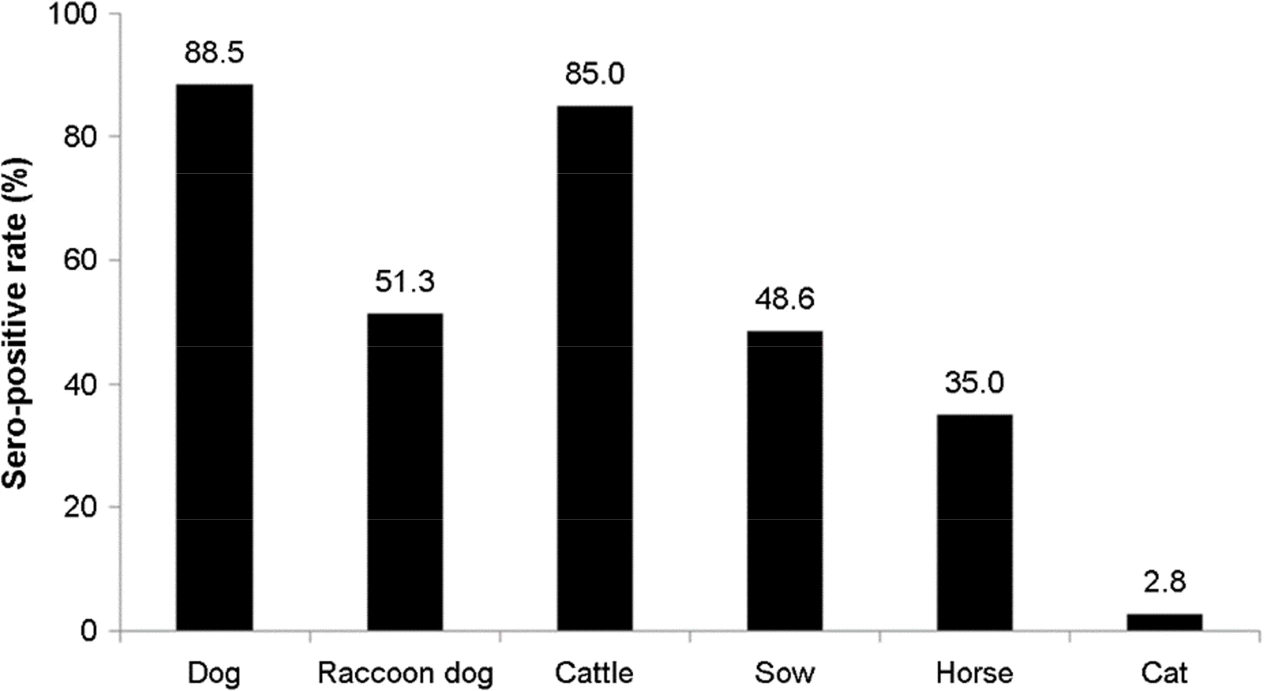
Table 1.
Distribution of CAV-2 virus neutralization antibody titers in dogs, raccoon dogs, horses, cattle, sows, and cats




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download