Abstract
In the present study we evaluated the anti-inflammatory potential of 3-hydroxy-4,7-megastigmadien-9-one (Comp) isolated from Ulva pertusa Kjellman, in LPS-stimulated bone marrow-derived dendritic cells (BMDCs). Comp treatment exhibited strong dose dependent inhibition of IL-12 p40 and IL-6 cytokine production with IC50 values of 7.85 ± 0.32 and 7.86 ± 0.18, respectively in LPS-stimulated BMDCs. Treatment of Comp inhibited MAPKs and NF-κB pathways in LPS-stimulated BMDCs by inhibiting the phosphorylation of ERK1/2, JNK1/2, p38 and IκB. Thus, these results suggest that Comp have a significant anti-inflammatory property and affirm further studies concerning the potentials of Comp for medicinal use.
REFERENCES
1). Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013; 24:631–44.

2). Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis associated colorectal cancer susceptibility. PLoS One. 2009; 4:e6026.
3). Honstettre A, Ghigo E, Moynault A, Capo C, Toman R, Akira S, et al. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through toll-like receptor 4. J Immunol. 2004; 172:3695–703.
4). Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptor in spontaneous commensal-dependent colitis. Immunity. 2006; 25:319–29.
5). Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptor is required for intestinal homeostasis. Cell. 2004; 118:229–41.
6). Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996; 14:397–440.

7). Ma J, Bang BR, Lu J, Eun SY, Otsuka M, Croft M, et al. The TNF family member 4-1BBL sustains inflammation by interacting with TLR signaling components during late-phase activation. Sci Signal. 2013; 6:ra87.

8). Shi Q, Cheng L, Liu Z, Hu K, Ran J, Ge D, et al. The p38 MAPK inhibitor SB203580 differentially modulates LPS-induced interleukin 6 expression in macrophages. Cent Eur J Immunol. 2015; 40:276–82.

9). Intayoung P, Limtrakul P, Yodkeeree S. Anti-inflammatory activities of crebanine by inhibition of NF-κB and AP-1 activation through suppressing MAPKs and Akt signaling in LPS-induced RAW 264.7 macrophages. Biol Pharm Bull. 2016; 39:54–61.
10). Tsumagari K, Abd Elmageed ZY, Sholl AB, Friedlander P, Abdraboh M, Xing M, et al. Simultaneous suppression of the MAP kinase and NF-κB pathways provides a robust therapeutic potential for thyroid cancer. Cancer Lett. 2015; 368:46–53.

11). Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokines expression. Pharmacol Res. 2004; 49:433–9.
12). Khan MN, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, et al. Anti-inflammatory activities of methanol extracts from various seaweed species. J Environ Biol. 2008; 29:465–9.
13). Shi J, Cheng C, Zhao H, Jing J, Gong N, Lu W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron (III) complex. Int J Biol Macromol. 2013; 60:341–6.
14). Hong JK, Bong MH, Park JC, Moon HK, Kim DW, Lee SC, et al. Antioxidant and immunomodulatory effects of Ulva pertusa Kjellman on broiler chickens. J Anim Sci Tech. 2011; 53:419–28.
15). Machida K, Kikuchi M. Norisoprenoids from Viburnum dilatatum. Phytochemistry. 1996; 5:1333–6.
16). Manzoor Z, Mathema VB, Chae D, Yoo ES, Kang HK, Hyun JW, et al. Extracts of the seaweed Sargassum macrocarpum inhibit the CpG-induced inflammatory response by attenuating the NF-κB pathway. Food Sci Biotechnol. 2014; 23:293–7.
17). Chae D, Manzoor Z, Kim SC, Kim S, Oh TH, Yoo ES, et al. Apo-9′-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar Drugs. 2013; 11:3272–87.
18). Manzoor Z, Kim S, Chae D, Yoo ES, Kang HK, Hyun JW, et al. Sea lettuce (Ulva fasciata) extract has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived macrophages and dendritic cells. Food Sci Biotechnol. 2013; 22:781–6.
19). Palona I, Namba H, Mitsutake N, Starenki D, Podtcheko A, Sedliarou I, et al. BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology. 2006; 147:5699–707.
Figure 1.
Effects of Comp on cell viability of BMDCs. BMDCs were treated with Comp (1~50 μM) for 18 h and viability was measured using MTT assay. Data are representative of three independent experiments.
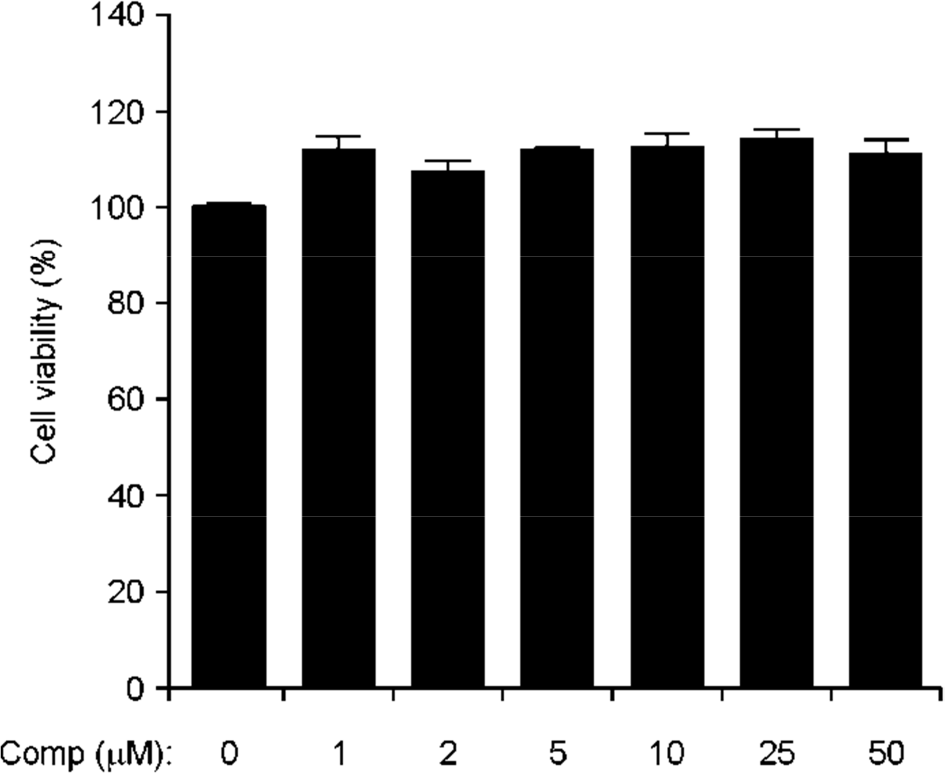
Figure 2.
Inhibitory effects of Comp on pro-inflammatory cytokine production in LPS-stimulated BMDCs. BMDCs were treated with Comp at the indicated doses for 1 h before stimulation with LPS (10 ng/ml). Enzyme-linked immunosorbent assay (ELISA) was used to measure the concentrations of murine IL-12 p40 (A), IL-6 (B), and TNF-α (C) in the culture supernatants. Data are representative of three independent experiments. Comp, 3-hydroxy-4,7-megastigmadien-9-one. ∗ p < 0.05, ∗∗ p < 0.01 vs. Comp-untreated cells in the presence of LPS.
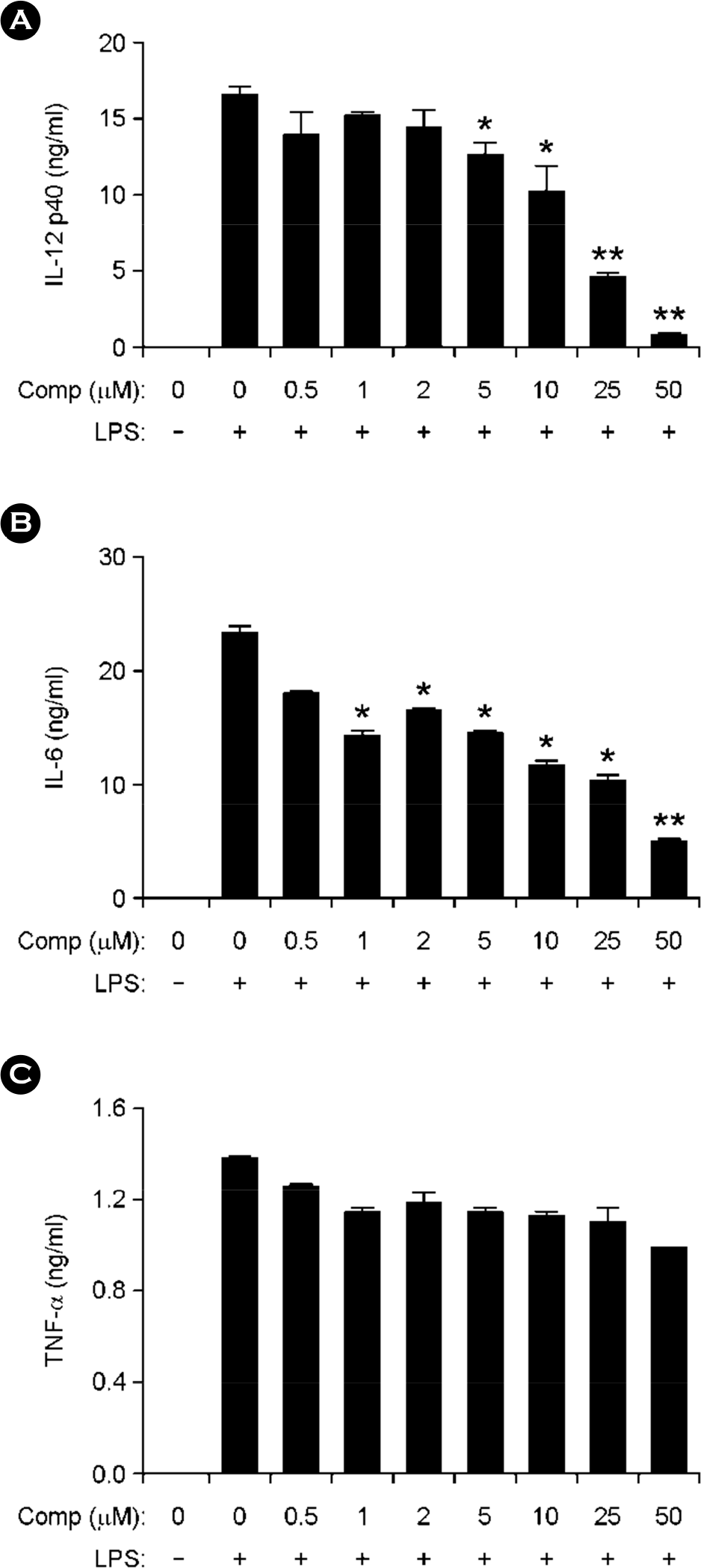
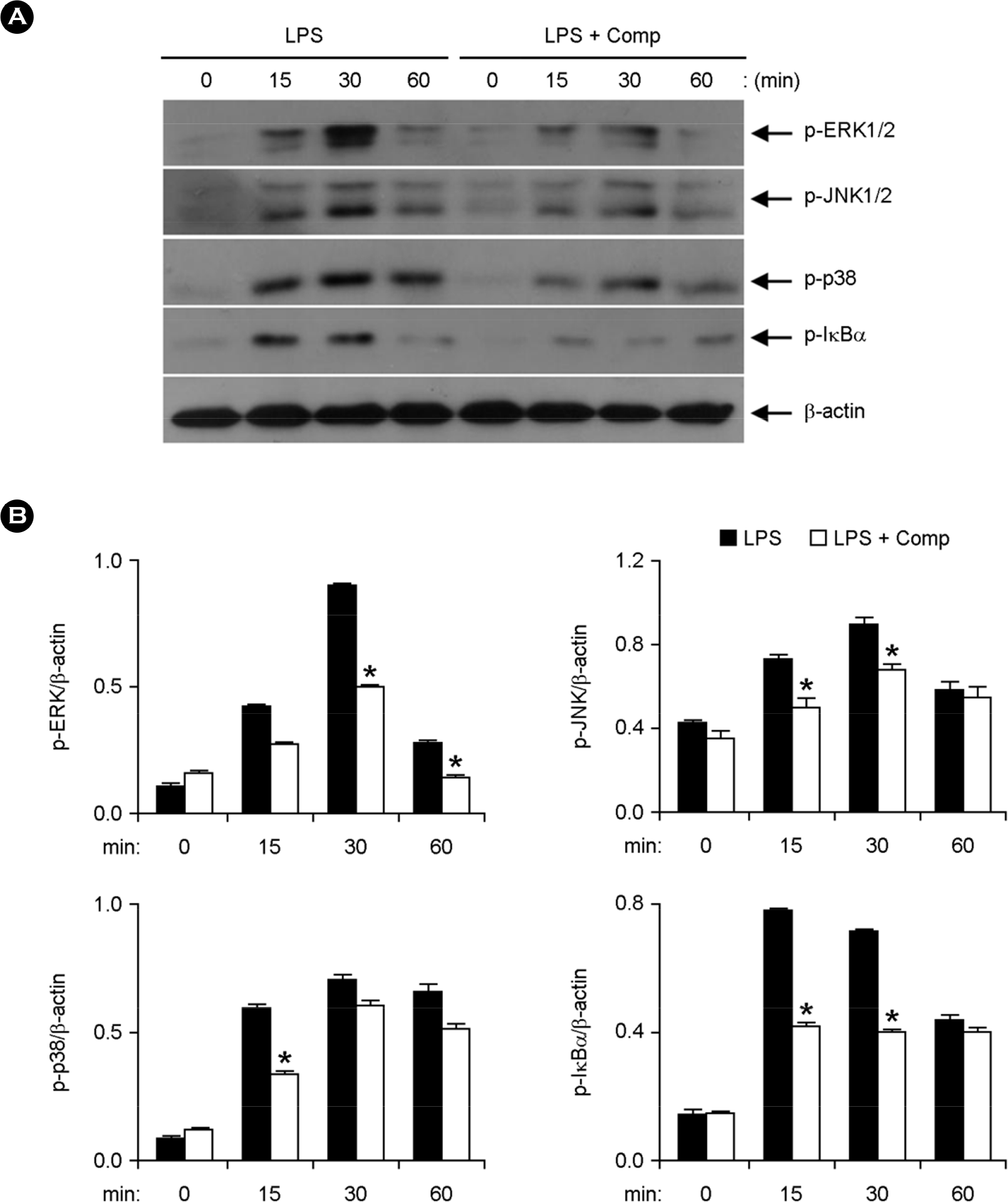
Figure 3.
Effects of Comp on the phosphorylation of MAPK and IκBα in LPS-stimulated BMDCs. (A) Cells were pre-treated with or without Comp (50 μM) for 1 h before stimulation with LPS (10 ng/ml). Total cell lysate was obtained at the indicated time intervals. Western blot analysis was performed on the cell lysate to assess phosphorylation of ERK, JNK, p38 and IκBα. β-actin was taken as the loading control. Data are representative of three independent experiments. (B) Phosphorylation of ERK, JNK, p38 and IκBα was quantified using scanning densitometry, and the band intensities were normalized by that of β-actin. Comp, 3-hydroxy-4,7-megastigmadien-9-one. ∗ p < 0.05 vs. Comp-untreated cells in the presence of LPS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download