Abstract
The effect of DMSO and sodium butyrate on the production of recombinant hepatitis A virus (HAV) capsid protein VP1 was evaluated and optimized in the culture of stably transfected Drosophila melanogaster S2 cells using culture plates and spinner flasks. The effect of DMSO and sodium butyrate was also evaluated to improve the recombinant VP1 production in stably transfected Drosophila S2 cells. A production level of 0.88 mg of recombinant VP1/liter was obtained in the culture-plate culture of stably transfected S2 cells at 6 days after induction with 0.5 mM CuSO4. The supplements of 2% DMSO and 10 mM sodium butyrate at 4 days post-inoculation increased recombinant VP1 accumulation by 141 and 104%, respectively, resulting in 2.17 and 1.7 mg/liter of recombinant VP1 production. In spinner flasks, recombinant VP1 production reached maximum level at 9 days after induction with 0.5 mM CuSO4, with approximately 4.96 mg/liter of recombinant VP1 production level. When 2% DMSO or 10 mM sodium butyrate was added at 5 days post-inoculation, the recombinant VP1 production was increased to 8.35 and 5.85 mg/liter, respectively. However, the synergistic effects of DMSO and sodium butyrate were not observed. These results indicate that DMSO and/or sodium butyrate can be successfully used to improve the recombinant HAV VP1 production in culture plates and spinner flasks.
Hepatitis A virus (HAV) causes one of the most acute viral hepatitis diseases in human and is classified as a picornavirus with a single-stranded, positive-sense RNA genome coding a single polyprotein that is subsequently processed into structural and nonstructural proteins. The structural proteins of HAV are divided into the capsid polypeptides VP1, VP2, VP3, and VP4. VP1 appears to be the dominant structural protein among the capsid proteins, containing a major immunodominant epitope of HAV from studies of the reaction of HAV with monoclonal antibodies and the use of isolated structural proteins or synthetic peptides for induction of neutralizing antibodies (1~4). In comparison with formalin-inactivated HAV vaccines, recombinant subunit vaccines comprised of antigenic peptides (epitopes) is a particularly attractive strategy for wide-scale immunization. Recombinant subunit vaccines are much safer and often more easily produced while offering potentially equivalent efficacy (5). Therefore, the production of large quantities of soluble HAV VP1 protein is necessary for immunological and biological studies directed towards vaccine development. Recombinant VP1 proteins were expressed in Escherichia coli solely and in fusion with the TrpE protein (anthranilate synthetase component I) or β-galactosidase (6~8). However, the presence of insoluble antigenic materials reduced the antigenicity of recombinant VP1 or the fused VP1 protein in an ELISA (6). HAV VP1 was also expressed in baculovirus-infected Spodoptera frugiperda (fall armyworm) cells as a fusion protein composed of VP1 and portions of VP3 and P2 (9). Although recombinant proteins retained antigenicity on immunoblots both to human HAV convalescent sera and to sera of rabbits immunized with HAV, recombinant proteins existed as an insoluble aggregate in the cytoplasm of infected insect cells. In our previous work, we generated stably transfected Drosophila melanogaster (fruitfly) S2 (Schneider 2) cells expressing recombinant VP1 (10). Recombinant VP1 was successfully secreted into a culture medium and the production of specific IgA in the small intestine was elicited by oral immunization and production of specific IgG in the serum by intraperitoneal immunization. It was suggested that recombinant VP1 from transfected Drosophila S2 cells could be used as an effective experimental immunogen in vaccine development research. However, recombinant VP1 production from Drosophila S2 cell systems has not yet been fully optimized. In this study, to improve recombinant VP1 production from stably transfected Drosophila S2 cells, we optimized the production of recombinant VP1 in culture plates and spinner flasks and investigated the effect of dimethylsulfoxide (DMSO) and sodium butyrate on recombinant VP1 production.
Stably transfected Drosophila melanogaster Schneider 2 (S2) cells expressing recombinant VP1 (10) were grown at 27℃ in T-25 flasks (Nunc, Denmark) in HyClone SFX-Insect medium (Thermo Scientific, Waltham, MA, USA) containing 300 µg/ml hygromycin B. Cells of 3 × 106 were inoculated into 6-well culture plates (culture plates) containing 1 ml of culture medium (an initial cell density: 3 × 106 cells/ml) and cultured for 6 days to analyze cell growth and recombinant VP1 expression. The expression of recombinant VP1 was induced by addition of 0.5 mM CuSO4 after the start of the run. In an experiment to determine the effects of DMSO and sodium butyrate on the expression of recombinant VP1, DMSO and a concentrated sodium butyrate solution were added to the cultures at 0, 2 and 4 days after CuSO4 induction. The cultures were centrifuged at 1,000 × g for 5 min and the supernatants were used to identify extracellular recombinant VP1 proteins. Protein concentrations were determined using an RC Bio-Rad assay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol.
Stably transfected Drosophila S2 cells expressing recombinant VP1 were cultured in spinner flasks (100 ml vol.; Bellco, Vineland, NJ) at an agitation rate of 40 rpm. Cells of 1.5 × 108 were inoculated in 50 ml of culture medium containing 25 µg/ml hygromycin B (an initial cell density: 3 × 106 cells/ml) and cultured for 10 days. In an experiment to determine the effects of DMSO and sodium butyrate on the expression of recombinant VP1, DMSO and a concentrated sodium butyrate solution were added to the cultures at 5 days after CuSO4 induction. The culture samples were centrifuged at 1,000 × g for 5 min and the supernatants were used to identify extracellular recombinant VP1 proteins.
Protein samples were separated by electrophoresis on 8% polyacrylamide-SDS gels (11), and analyzed using Western blot analysis. The electrophoresed proteins on the gel were transferred onto nitrocellulose membrane (Amersham Pharmacia, Piscataway, NJ, USA), blocked with blocking solution [3% (w/v) non-fat dried skim milk powder in TBS (Tris-buffered saline; 20 mM Tris-HCl, 500 mM NaCl, pH 7.9)], incubated with mouse anti-V5 (1:2,000 dilution in blocking solution), and probed with alkaline phosphatase-conjugated goat anti-mouse IgG (1:1,000 dilution in blocking solution). The membranes were washed and BCIP (5-bromo-4-chloroindol-3-yl phosphate/NBT (nitro blue tetrazolium) solution (Amresco, Solon, OH, USA) was added. The reaction was quenched with distilled water. VP1 band intensities were quantitated by densitometry analysis using the imaging TINA 2.0 software.
In our previous work, we generated stably transfected Drosophila S2 cells expressing recombinant HAV VP1 protein (10). Recombinant VP1 was secreted into a medium with a molecular size of 42~49 kDa, due to glycosylation. The secreted recombinant VP1 (VP1 in medium fraction) was approximately 90% of the total VP1 production.
Dimethylsulfoxide (DMSO) has been known to be an effective permeabilizing agent that helps the release of intracellular products from plant cells (12) as well as a stabilizing agent for proteins (13). DMSO treatment can have a stimulatory effect on protein expression in animal cells (14, 15) while sodium butyrate has been known to increase recombinant protein expression in animal cells (16, 17). Although the mechanism of DMSO and sodium butyrate-mediated induction of protein expression is not clearly defined, DMSO is suggested to improve recombinant protein production by increasing the stability of the recombinant protein or by increasing transcription efficiency (18) whereas sodium butyrate is suggested to cause changes in the chromatin structure by histone hyper-acetylation in animal cells, which seems to be correlated with a modulation of gene expression (19, 20). DMSO and sodium butyrate have been reported to increase recombinant proteins such as rotavirus VP7 and cyclooxygenase 2 in T-flask cultures of stably transfected Drosophila S2 cells (18, 21). These findings suggest that DMSO or sodium butyrate treatment may improve the production of recombinant hepatitis A virus capsid protein VP1 in stably transfected insect cells.
To improve the production of recombinant VP1 from stably transfected S2 cells, the effects of DMSO and sodium butyrate on cell growth and recombinant VP1 production were investigated in culture plates. Stably transfected S2 cells were cultured for six days at an initial density of 3 × 106 cells/ml and the expression of recombinant VP1 was induced by addition of 0.5 mM CuSO4 after the start of the run. DMSO (0.5, 1, 1.5, 2, 2.5 or 3%) or sodium butyrate (2.5, 5, 10, 15 or 20 mM) was added to the cultures at zero, two and four days post-inoculation so that the stably transfected S2 cells were incubated with DMSO or sodium butyrate for six, four and two days, respectively. The DMSO supplement inhibited cell growth but increased recombinant VP1 production in medium fractions (Table 1). Recombinant VP1 accumulation reached maximum level with a supplement of 2% DMSO at four days post-inoculation. At this peak, recombinant VP1 production was 141% higher than control, which is between the 80% increase reported for recombinant rotavirus VP7 production in stably transfected D. melanogaster S2 cells (21) and the 170% increase for recombinant cyclooxygenase 2 (18). The sodium butyrate supplement also inhibited cell growth but increased VP1 production (Table 2). Maximum VP1 production of 104% higher as compared to the control, which is higher than the 60 and 86% increase reported for recombinant rotavirus VP7 and cyclooxygenase 2 production in stably transfected S2 cells (18, 21), was reached at four days post-inoculation with the 10 mM sodium butyrate supplement.
The synergy of DMSO and sodium butyrate on recombinant VP1 production was also investigated (Fig. 1). Both DMSO (2%) and sodium butyrate (10 mM) were added to the cultures at four days post-inoculation at an initial cell density of 3 × 106 cells/ml. As a control, either DMSO (2%) or sodium butyrate (10 mM) was added at four days post-inoculation to cultures under the same condition. The recombinant VP1 productions were determined after six days after incubations. A production level of 0.88 mg of recombinant VP1/liter was obtained in culture-plate culture of stably transfected S2 cells six days after induction with 0.5 mM CuSO4, based on Western blot analysis using recombinant VP1 purified from stably transfected S2 cells (Fig. 1A and B), which is less than the maximum production level of 6.24 mg of recombinant VP1/liter obtained in T-flask culture (10). In our previous T-flask culture, stably transfected S2 cells of an initial density of 5 × 106 cells/ml that were cultured in Shields and Sang M3 insect medium containing 10% (v/v) insect medium supplement (IMS), resulted in the maximum production level of 6.24 mg of recombinant VP1/liter obtained in a T-flask culture of Drosophila S2 cells five days after induction with 0.5 mM CuSO4. Therefore, decrease in recombinant VP1 production in culture plates was probably due to the differences in culture medium and initial cell density. DMSO and sodium butyrate increased the recombinant VP1 production to 2.17 and 1.7 mg/liter, respectively (Fig. 1A and B). In comparisons to DMSO only, supplementation with both DMSO and sodium butyrate resulted in lower recombinant VP1 production; there was no synergistic interaction between DMSO and sodium butyrate.
The stably transfected S2 cells were also cultured in spinner flasks to increase the recombinant VP1 production. The S2 cells of initial density of 3 × 106 cells/ml were inoculated in 100 ml spinner flasks containing 50 ml culture medium and recombinant VP1 production was induced by the addition of 0.5 mM CuSO4 at zero day. DMSO or sodium butyrate was added at five days after CuSO4 induction; the addition of DMSO and sodium butyrate resulted in slight inhibition of cell growth (Fig. 2), whereas recombinant VP1 production was increased by DMSO and sodium butyrate (Table 3), reaching the maximum at nine days after CuSO4 induction. The presence of 2% DMSO and 10 mM sodium butyrate separately increased the recombinant VP1 production by 63.3 and 13.2%, respectively. Moreover, recombinant VP1 production was lower when supplemented with both 2% DMSO and 10 mM sodium butyrate than with 2% DMSO only, suggesting no synergistic interaction between DMSO and sodium butyrate. The production level of recombinant VP1 in spinner flasks was about 4.96 mg/liter at 9 days after CuSO4 induction, based on Western blot analysis using recombinant VP1 purified from stably transfected S2 cells (Fig. 3). The DMSO and sodium butyrate treatments increased the recombinant VP1 production levels to 8.35 and 5.85 mg/liter, respectively. These levels are much higher than obtained in culture plates. A level of 8.35 mg/liter is also better than our previously reported expression level (6.24 mg/liter) for Drosophila S2 cells (10).
In conclusion, the effect of DMSO and sodium butyrate was evaluated to improve the recombinant VP1 production in stably transfected Drosophila S2 cells. The supplementation of DMSO and sodium butyrate dramatically increased the recombinant VP1 production in culture plates and spinner flasks.
Figures and Tables
Figure 1
Recombinant VP1 production in DMSO and/or sodium butyrate-supplemented culture of stably transfected S2 cells using culture plates. (A) DMSO (2%) and sodium butyrate (10 mM) were added to the cultures at 4 days post-inoculation. After 2 days incubation, recombinant VP1 expressions in medium fractions were confirmed by Western blot analysis. M: molecular weight marker, Lane 1: the medium fraction of non-treated S2 cells, Lane 2: the medium fraction of 2% DMSO-treated S2 cells, Lane 3: the medium fraction of 10 mM sodium butyrate-treated S2 cells, Lane 4: the medium fraction of 2% DMSO and 10 mM sodium butyrate-treated S2 cells, Lane 5~8: 50, 100, 250, 500 ng of purified recombinant VP1 control from S2 cells. (B) The contents of recombinant VP1 from (A) experiment were estimated and are plotted as a bar diagram. Data are reported as mean ± S.D. Statistically significant differences between treatment and control groups were determined using Student's t test (** p < 0.01).
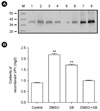
Figure 2
Cell growth in DMSO and/or sodium butyrate-supplemented culture of stably transfected S2 cells using spinner flasks. Data are reported as mean ± S.D. Statistically significant differences between treatment and control groups were determined using Student's t test (* p < 0.05, *** p < 0.001).

Figure 3
Recombinant VP1 production in DMSO and/or sodium butyrate-supplemented culture of stably transfected S2 cells using spinner flasks. (A) DMSO (2%) and sodium butyrate (10 mM) were added to the cultures at 5 days post-inoculation. Recombinant VP1 expressions in medium fractions at 9 days post-inoculation were confirmed by Western blot analysis. Lane 1: the medium fraction of non-treated S2 cells, Lane 2: the medium fraction of 2% DMSO-treated S2 cells, Lane 3: the medium fraction of 10 mM sodium butyrate-treated S2 cells, Lane 4: the medium fraction of 2% DMSO and 10 mM sodium butyrate-treated S2 cells, Lane 5-8: 50, 100, 250, 500 ng of purified recombinant VP1 control from S2 cells. (B) The contents of recombinant VP1 from (A) experiment were estimated and are plotted as a bar diagram. Data are reported as mean ± S.D. Statistically significant differences between treatment and control groups were determined using Student's t test (* p < 0.05).

References
1. Hughes JV, Stanton LW, Tomassini JE, Long WJ, Scolnick EM. Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol. 1984. 52:465–473.

2. Hughes JV, Stanton LW. Isolation and immunizations with hepatitis A viral structural proteins: induction of antiprotein, antiviral, and neutralizing responses. J Virol. 1985. 55:395–401.

3. Stapleton JT, Lemon SM. Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol. 1987. 61:491–498.

4. Emini EA, Hughes JV, Perlow DS, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985. 55:836–839.

6. Ostermayr R, von der Helm K, Gauss-Müller V, Winnacker EL, Deinhardt F. Expression of hepatitis A virus cDNA in Escherichia coli: antigenic VP1 recombinant proteins. J Virol. 1987. 61:3645–3647.

7. Johnston JM, Harmon SA, Binn LN, Richards OC, Ehrenfeld E, Summers DF. Antigenic and immunogenic properties of a hepatitis A virus capsid protein expressed in Escherichia coli. J Infect Dis. 1988. 157:1203–1211.

8. Gauss-Muller V, Zhou MQ, von der Helm K, Deinhardt F. Recombinant proteins VP1 and VP3 of hepatitis A virus prime for neutralizing response. J Med Virol. 1990. 31:277–283.

9. Harmon SA, Johnston JM, Ziegelhoffer T, Richards OC, Summers DF, Ehrenfeld E. Expression of hepatitis A virus capsid sequences in insect cells. Virus Res. 1988. 10:273–280.

10. Lee JM, Lee HH, Hwang-Bo J, Shon DH, Kim W, Chung IS. Expression and immunogenicity of recombinant polypeptide VP1 of human hepatitis A virus in stably transformed fruitfly (Drosophila melanogaster) Schneider 2 cells. Biotechnol Appl Biochem. 2009. 53:101–109.

11. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.

12. Schmidt AJ, Lee JM, An G. Media and environmental effects on phenolics production from Tobacco cell cultures. Biotechnol Bioeng. 1989. 33:1437–1444.

13. Wahl MF, An GH, Lee JM. Effects of dimethyl sulfoxide on heavy chain monoclonal antibody production from plant cell culture. Biotechnol Lett. 1995. 17:463–468.

14. Rodriguez J, Spearman M, Huzel N, Butler M. Enhanced production of monomeric interferon-beta by CHO cells through the control of culture conditions. Biotechnol Prog. 2005. 21:22–30.

15. Li J, Sun X, Zhang Y. Improvement of hepatitis B surface antigen expression by dimethyl sulfoxide in the culture of recombinant Chinese hamster ovary cells. Process Biochem. 2006. 41:317–322.

16. Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol. 1991. 19:35–47.

17. Oster T, Thioudellet C, Chevalot I, Masson C, Wellman M, Marc A, et al. Induction of recombinant human gamma-glutamyl transferase by sodium butyrate in transfected V79 and CHO Chinese hamster cells. Biochem Biophys Res Commun. 1993. 193:406–412.

18. Chang KH, Park JH, Lee YH, Kim JH, Chun HO, Kim JH, et al. Dimethylsulfoxide and sodium butyrate enhance the production of recombinant cyclooxygenase 2 in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett. 2002. 24:1353–1359.
19. Oh SK, Vig P, Chua F, Teo WK, Yap MG. Substantial overproduction of antibodies by applying osmotic pressure and sodium butyrate. Biotechnol Bioeng. 1993. 42:601–610.

20. Arts J, Lansink M, Grimbergen J, Toet KH, Kooistra T. Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem J. 1995. 310:171–176.

21. Park JH, Chang KH, Lee YH, Kim HY, Yang JM, Chung IS. Production of recombinant rotavirus capsid protein VP7 from stably transformed Drosophila melanogaster S2 cells. J Microbial Biotechnol. 2002. 12:563–568.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download