Abstract
Despite decreasing Helicobacter pylori prevalence, the prevalence of peptic ulcer disease is increasing in the aged population, mainly due to increasing use of NSAIDs to manage pain and inflammation. In addition, low dose aspirin is employed as an anti-coagulant for those who have suffered or are at high risk of ischemic stroke and cardiovascular disease. However, NSAIDs and aspirin are injurious to mucosa of stomach and duodenum. NSAID-induced inhibition of mucosal prostaglandin synthesis is thought to be a major mechanism of gastrointestinal mucosal injury. The proportion of elderly has increased rapidly in Korea, with the proportion over 65 years old expected to be 24.3% in 2030. In this higher-risk population, the strategy to reduce the incidence of NSAID-related peptic ulcers and complications such as bleeding, obstruction and perforation is very important. Proton pump inhibitors (PPIs) with cyclooxygenase-2 inhibitor can be used for reducing the risk of NSAID-related ulcers and upper gastrointestinal (GI) complications. However, continuous use of PPI has several problems. In addition, NSAID-re-lated problems in the lower GI tract have increased, in contrast to the decrease of NSAID-related upper GI disease. The aim of this review is to provide an evidence-based knowledge regarding the mechanism, complications of treatment, and prevention strategies for NSAID- or aspirin-related peptic ulcer disease in Korea.
References
1. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009; 29:938–946.

2. Wang YR, Richter JE, Dempsey DT. Trends and outcomes of hospitalizations for peptic ulcer disease in the United States, 1993 to 2006. Ann Surg. 2010; 251:51–58.

3. Wang AY, Peura DA. The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest Endosc Clin N Am. 2011; 21:613–635.
4. Manuel D, Cutler A, Goldstein J, Fennerty MB, Brown K. Decreasing prevalence combined with increasing eradication of Helicobacter pylori infection in the United States has not resulted in fewer hospital admissions for peptic ulcer disease-related complications. Aliment Pharmacol Ther. 2007; 25:1423–1427.
5. Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: a nationwide study in The Netherlands. Aliment Pharmacol Ther. 2006; 23:1587–1593.

6. Kim JI, Kim SG, Kim N, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Changing prevalence of upper gastrointestinal disease in 28 893 Koreans from 1995 to 2005. Eur J Gastroenterol Hepatol. 2009; 21:787–793.
7. Bae S, Kim N, Kang JM, et al. Incidence and 30-day mortality of peptic ulcer bleeding in Korea. Eur J Gastroenterol Hepatol. 2012; 24:675–682.

8. Bae S, Shim KN, Kim N, et al. Incidence and short-term mortality from perforated peptic ulcer in Korea: a population-based study. J Epidemiol. 2012; 22:508–516.

9. Newton JL. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004; 125:867–870.

10. Kang JM, Kim N, Kim JH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G1147–G1153.

11. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

12. Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012; 11:996–1004.

13. Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009; 8:311–323.

14. Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008; 20:185–196.

15. Majumdar AP. Regulation of gastrointestinal mucosal growth during aging. J Physiol Pharmacol. 2003; 54(Suppl 4):143–154.
16. Jo HJ, Kim N, Nam RH, et al. The effect of cochinchina momordica seed extract on gastric acid secretion and morphologic change in aged rat stomach. Gut Liver. 2013; 7:560–568.

17. Salles N. Is stomach spontaneously ageing? Pathophysiology of the ageing stomach. Best Pract Res Clin Gastroenterol. 2009; 23:805–819.

18. Park KS. Aging and digestive diseases: at the view of the functional change of gastrointestinal tract. Korean J Gastroenterol. 2011; 58:3–8.

19. Kwon JH, Choi MG, Lee SW, et al. Trends of gastrointestinal diseases at a single institution in korea over the past two decades. Gut Liver. 2009; 3:252–258.

20. Kim HG, Lee CK, Cho SM, et al. Neuregulin 1 up-regulates the expression of nicotinic acetylcholine receptors through the ErbB2/ErbB3-PI3K-MAPK signaling cascade in adult autonomic ganglion neurons. J Neurochem. 2013; 124:502–513.

21. Kim JJ, Kim N, Lee BH, et al. Risk factors for development and recurrence of peptic ulcer disease. Korean J Gastroenterol. 2010; 56:220–228.

22. Kim JJ, Kim N, Park HK, et al. Clinical characteristics of patients diagnosed as peptic ulcer disease in the third referral center in 2007. Korean J Gastroenterol. 2012; 59:338–346.

23. Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000; 119:521–535.

24. Aalykke C, Lauritsen K. Epidemiology of NSAID-related gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001; 15:705–722.

25. Wong GL, Wong VW, Chan Y, et al. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009; 137:525–531.
26. Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987; 28:527–532.

27. Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 1996; 156:1530–1536.

28. Kang JM, Seo PJ, Kim N, et al. Analysis of direct medical care costs of peptic ulcer disease in a Korean tertiary medical center. Scand J Gastroenterol. 2012; 47:36–42.

29. Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007; 76:1005–1012.
30. Milosavljevic T, Kostić-Milosavljević M, Jovanović I, Krstić M. Complications of peptic ulcer disease. Dig Dis. 2011; 29:491–493.

31. Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther. 2009; 30:517–531.

32. Christensen S, Riis A, Nørgaard M, Sørensen HT, Thomsen RW. Short-term mortality after perforated or bleeding peptic ulcer among elderly patients: a population-based cohort study. BMC Geriatr. 2007; 7:8.

33. Kocer B, Surmeli S, Solak C, et al. Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol. 2007; 22:565–570.

34. Jang HJ, Choi MH, Shin WG, et al. Has peptic ulcer disease changed during the past ten years in Korea? A prospective multicenter study. Dig Dis Sci. 2008; 53:1527–1531.

35. Peterson WL, Ciociola AA, Sykes DL, McSorley DJ, Webb DD. Ranitidine bismuth citrate plus clarithromycin is effective for healing duodenal ulcers, eradicating H. pylori and reducing ulcer recurrence. RBC H. pylori Study Group. Aliment Pharmacol Ther. 1996; 10:251–261.
36. Lee JH, Lee YC, Jeon SW, et al. Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 2009; 54:309–317.

37. Jo YW, Choi JY, Ha CY, Min HJ, Lee OJ. The clinical features and prognostic factors of nonvariceal upper gastrointestinal bleeding in the patients with liver cirrhosis. Korean J Helicobacter Up Gastrointest Res. 2013; 13:235–242.

38. Schlansky B, Hwang JH. Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy. J Gastroenterol. 2009; 44(Suppl 19):44–52.

39. Sinha M, Gautam L, Shukla PK, Kaur P, Sharma S, Singh TP. Current perspectives in NSAID-induced gastropathy. Mediators Inflamm. 2013. DOI: doi:10.1155/2013/258209.

40. Wallace JL. NSAID gastropathy and enteropathy: distinct pathogenesis likely necessitates distinct prevention strategies. Br J Pharmacol. 2012; 165:67–74.

41. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999; 117:17–25.

42. Iwamoto J, Saito Y, Honda A, Matsuzaki Y. Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World J Gastroenterol. 2013; 19:1673–1682.

43. Tomisato W, Tanaka K, Katsu T, et al. Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem Biophys Res Commun. 2004; 323:1032–1039.

44. Somasundaram S, Rafi S, Hayllar J, et al. Mitochondrial damage: a possible mechanism of the "topical" phase of NSAID induced injury to the rat intestine. Gut. 1997; 41:344–353.

45. Lichtenberger LM. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem Pharmacol. 2001; 61:631–637.
46. Tomisato W, Tsutsumi S, Rokutan K, Tsuchiya T, Mizushima T. NSAIDs induce both necrosis and apoptosis in guinea pig gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol. 2001; 281:G1092–G1100.

47. Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet. 1996; 348:1413–1416.

48. Estes LL, Fuhs DW, Heaton AH, Butwinick CS. Gastric ulcer perforation associated with the use of injectable ketorolac. Ann Pharmacother. 1993; 27:42–43.

49. Wolfe PA, Polhamus CD, Kubik C, Robinson AB, Clement DJ. Giant duodenal ulcers associated with the postoperative use of ketorolac: report of three cases. Am J Gastroenterol. 1994; 89:1110–1111.
50. Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues–is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 2009; 13:3753–3763.
51. Gudis K, Sakamoto C. The role of cyclooxygenase in gastric mucosal protection. Dig Dis Sci. 2005; 50(Suppl 1):S16–S23.

52. Konturek SJ, Konturek PC, Pawlik T, Sliwowski Z, Ochmański W, Hahn EG. Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J Physiol Pharmacol. 2004; 55(Suppl 2):5–17.
53. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993; 90:11693–11697.

54. Laine L. Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am. 1996; 6:489–504.

55. Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983; 245:G601–G623.

56. Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994; 91:12013–12017.

57. Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994; 4:17–23.
58. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Longterm Arthritis Safety Study. JAMA. 2000; 284:1247–1255.
59. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001; 15:691–703.

60. MacDonald TM, Morant SV, Goldstein JL, Burke TA, Pettitt D. Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxibs, and older, non-specific non-steroidal anti-inflammatory drugs. Gut. 2003; 52:1265–1270.

61. Targownik LE, Metge CJ, Leung S, Chateau DG. The relative efficacies of gastroprotective strategies in chronic users of non-steroidal anti-inflammatory drugs. Gastroenterology. 2008; 134:937–944.

62. Sung JJ, Chan FK, Chen M, et al. Asia-Pacific Working Group. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011; 60:1170–1177.

63. Vaananen PM, Keenan CM, Grisham MB, Wallace JL. Pharmacological investigation of the role of leukotrienes in the pathogenesis of experimental NSAID gastropathy. Inflammation. 1992; 16:227–240.

64. Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993; 34:742–747.

65. Peskar BM. Role of leukotriene C4 in mucosal damage caused by necrotizing agents and indomethacin in the rat stomach. Gastroenterology. 1991; 100:619–626.

66. Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997; 112:1000–1016.

67. Song HJ, Kwon JW, Kim N, Park YS. Cost effectiveness associated with Helicobacter pylori screening and eradication in patients taking nonsteroidal anti-inflammatory drugs and/or aspirin. Gut Liver. 2013; 7:182–189.
68. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
69. Graham DY, Chan FK. NSAIDs, risks, and gastroprotective strategies: current status and future. Gastroenterology. 2008; 134:1240–1246.

70. Kim GH, Lee HJ. NSAID-induced gastropathy and H. pylori infection. Kim N, editor. Helicobacter pylori. Seoul: Daehan Medical Book Publishing;2015. p. 298–306.
71. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a metaanalysis. Lancet. 2002; 359:14–22.
72. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006; 4:130–142.
73. Wardi J, Shirin H. Non-steroidal anti-inflammatory drug-induced gastroduodenal toxicity and Helicobacter pylori. Isr Med Assoc J. 2003; 5:195–197.
74. Konturek JW, Dembinski A, Konturek SJ, Stachura J, Domschke W. Infection of Helicobacter pylori in gastric adaptation to continued administration of aspirin in humans. Gastroenterology. 1998; 114:245–255.
75. Lee BH, Kim N, Nam RH, et al. Difficult establishment of a chronic nonsteroidal anti-inflammatory drugs induced gastric inflammation rat model due to gastric adaptation and small bowel damage. Korean J Gastroenterol. 2014; 63:341–347.

76. Hunt RH, Bazzoli F. Review article: should NSAID/low-dose aspirin takers be tested routinely for H. pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther. 2004; 19(Suppl 1):9–16.
77. Vergara M, Catalán M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005; 21:1411–1418.
78. Kiltz U, Zochling J, Schmidt WE, Braun J. Use of NSAIDs and infection with Helicobacter pylori–what does the rheumatologist need to know? Rheumatology (Oxford). 2008; 47:1342–1347.
79. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
80. Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. International NSAID Consensus Group. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis–an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015; 13:55.

81. de Leest HT, Steen KS, Lems WF, et al. Eradication of Helicobacter pylori does not reduce the incidence of gastroduodenal ulcers in patients on long-term NSAID treatment: double-blind, randomized, placebo-controlled trial. Helicobacter. 2007; 12:477–485.
82. Lancaster-Smith MJ, Jaderberg ME, Jackson DA. Ranitidine in the treatment of non-steroidal anti-inflammatory drug associated gastric and duodenal ulcers. Gut. 1991; 32:252–255.

83. Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAID-Associated Gastric Ulcer Study Group. Arch Intern Med. 2000; 160:1455–1461.
84. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989; 320:69–75.

85. Hawkey CJ, Karrasch JA, Szczepañski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998; 338:727–734.
86. Wu CY, Wu MS, Chen CJ, Li MC, Lin JT, Chen GH. The interaction of H. pylori infection and NSAIDs in cyclooxygenase-2 mRNA expression in gastric antral, corpus mucosa, and gastric ulcer. J Clin Gastroenterol. 2005; 39:50–55.
87. Cheung DY, Jung HY, Song HJ, Jung SW, Jung HC. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Guidelines of treatment for non-bleeding peptic ulcer disease. Korean J Gastroenterol. 2009; 54:285–297.

88. Health and Social Care Information Centre. Prescription cost analysis. England 2006. Health and Social Care Information Centre;2007. [cited 2016 May 15]. Available from:. http://www.hscic.gov.uk/catalogue/PUB01307.
89. Federal Interagency Forum on Aging Related Statistics. Older Americans 2012: Key indicators of well-being. Washington, DC: US Government Printing Office;2012. [cited 2016 May 15]. Available from:. http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/Data_2012.aspx.
90. Chan FK, Wong VW, Suen BY, et al. Combination of a cyclo-oxy-genase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet. 2007; 369:1621–1626.

91. Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009; 104:728–738.

92. Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009; 104:1633–1641.

93. Zhao Y, Encinosa W. Hospitalizations for gastrointestinal bleeding in 1998 and 2006. HCUP Statistical Brief #65 [Internet]. Rockville, MD: Agency for Healthcare Research and Quality;Dec 2008. [cited 2016 May 15]. Available from:. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb65.pdf.
94. Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012; 107:1190–1195.

95. Cavallaro LG, Monica F, Germanà B, Marin R, Sturniolo GC, Saia M. Time trends and outcome of gastrointestinal bleeding in the Veneto region: a retrospective population based study from 2001 to 2010. Dig Liver Dis. 2014; 46:313–317.

96. Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002; 8(15 Suppl):S383–S391.
97. Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010; 69:1453–1458.

98. Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011; 9:38.

99. Greenberg JD, Bingham CO 3rd, Abramson SB, Reed G, Sebaldt RJ, Kremer J. Effect of cardiovascular comorbidities and concomitant aspirin use on selection of cyclooxygenase inhibitor among rheumatologists. Arthritis Rheum. 2005; 53:12–17.

100. Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010; 39:433–464.

101. Maiden L. Capsule endoscopic diagnosis of nonsteroidal anti-inflammatory drug-induced enteropathy. J Gastroenterol. 2009; 44(Suppl 19):64–71.

102. Endo H, Hosono K, Inamori M, et al. Incidence of small bowel injury induced by low-dose aspirin: a crossover study using capsule endoscopy in healthy volunteers. Digestion. 2009; 79:44–51.

103. Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005; 3:55–59.

104. Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005; 128:1172–1178.

105. Fujimori S, Gudis K, Takahashi Y, et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010; 40:504–510.

106. Scarpignato C. NSAID-induced intestinal damage: are luminal bacteria the therapeutic target? Gut. 2008; 57:145–148.

107. Shin CM, Kim N, Kim YS, et al. Impact of long-term proton pump inhibitor therapy on gut microbiota in F344 rats: pilot study. Gut Liver. 2016. [Epub ahead of print].

108. Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010; 8:504–508.

109. Compare D, Pica L, Rocco A, et al. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest. 2011; 41:380–386.

110. Laine L, Smith R, Min K, Chen C, Dubois RW. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2006; 24:751–767.

111. Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclooxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005; 365:475–481.

112. Bombardier C, Laine L, Reicin A, et al. VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000; 343:1520–1528.
113. ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials. 2006; 1:e33.
114. Sturkenboom MC, Burke TA, Dieleman JP, Tangelder MJ, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford). 2003; 42(Suppl 3):iii23–iii31.

115. Cai S, García Rodríguez LA, Massó-González EL, HernándezDíaz S. Uncomplicated peptic ulcer in the UK: trends from 1997 to 2005. Aliment Pharmacol Ther. 2009; 30:1039–1048.

116. Graham DY, White RH, Moreland LW, et al. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann Intern Med. 1993; 119:257–262.
117. Raskin JB, White RH, Jaszewski R, Korsten MA, Schubert TT, Fort JG. Misoprostol and ranitidine in the prevention of NSAID-induced ulcers: a prospective, double-blind, multicenter study. Am J Gastroenterol. 1996; 91:223–227.
118. Agrawal NM, Roth S, Graham DY, et al. Misoprostol compared with sucralfate in the prevention of nonsteroidal anti-inflammatory drug-induced gastric ulcer. A randomized, controlled trial. Ann Intern Med. 1991; 115:195–200.
119. Valentini M, Cannizzaro R, Poletti M, et al. Nonsteroidal antiinflammatory drugs for cancer pain: comparison between misoprostol and ranitidine in prevention of upper gastrointestinal damage. J Clin Oncol. 1995; 13:2637–2642.

120. Inman W. Drug safety research unit (Southampton). PEM News. 1991; 7:32–34.
121. Graham DY, Agrawal NM, Campbell DR, et al. NSAID-Associated Gastric Ulcer Prevention Study Group. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002; 162:169–175.
122. Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ. 2004; 329:948.

123. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006; 101:701–710.

124. Steinman MA, McQuaid KR, Covinsky KE. Age and rising rates of cyclooxygenase-2 inhibitor use. Results from a national survey. J Gen Intern Med. 2006; 21:245–250.
Fig. 1.
Two related isoforms of the COX and LOX enzyme pathway and the role of non-steroidal anti-inflammatory drug (NSAID). COX-1 is expressed constitutively. It regulates normal cellular processes such as gastric cytoprotection, vascular homeostasis, platelet aggregation, and kidney function. COX-2 is increased during states of inflammation or in response to mitogenic stimuli. Nonselective NSAID inhibits the COX-1 and COX-2 pathways. Selective COX-2 NSAIDs inhibit only the COX-2 pathway. When COX-1 and COX-2 are inhibited, its inhibitor, 5-LOX enzyme, is more activated and LTB4, C4 and D4 are more induced. COX, cylcooxygenase; PG, prostaglan-din; TX, thromboxane; LOX, lipoxyge-nase; LT, leukotriene; GI, gastrointestinal.
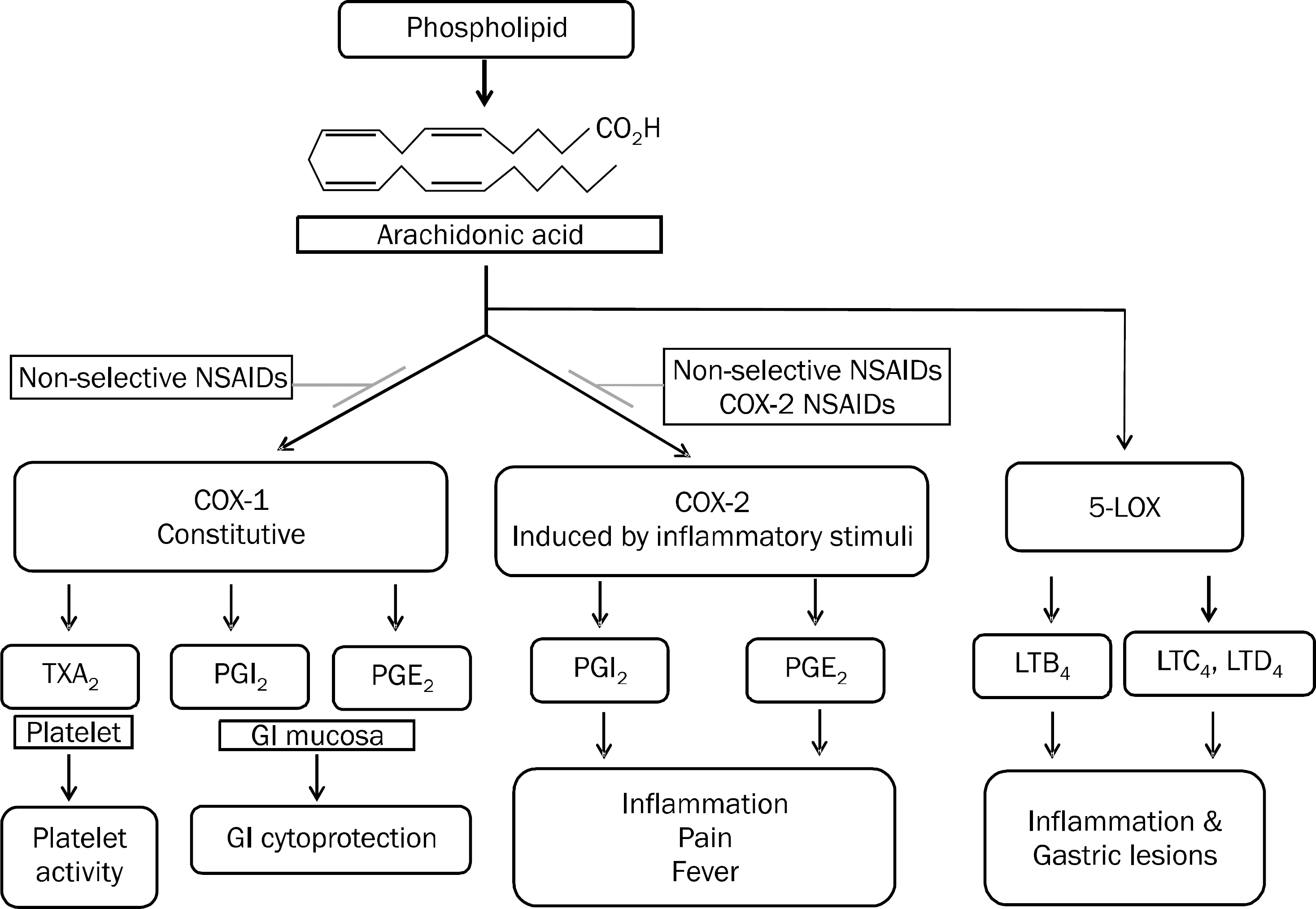
Fig. 2.
Algorithm for long-term NSAID therapy according to a patient's gastrointestinal (GI) and cardiovascular (CV) risk factors. ASA, aspirin; ns-NSAID, non-specific NSAID; PPI, proton pump inhibitor. Adapted from the article of Scarpignato et al.81 (BMC Med 2015;13:55).
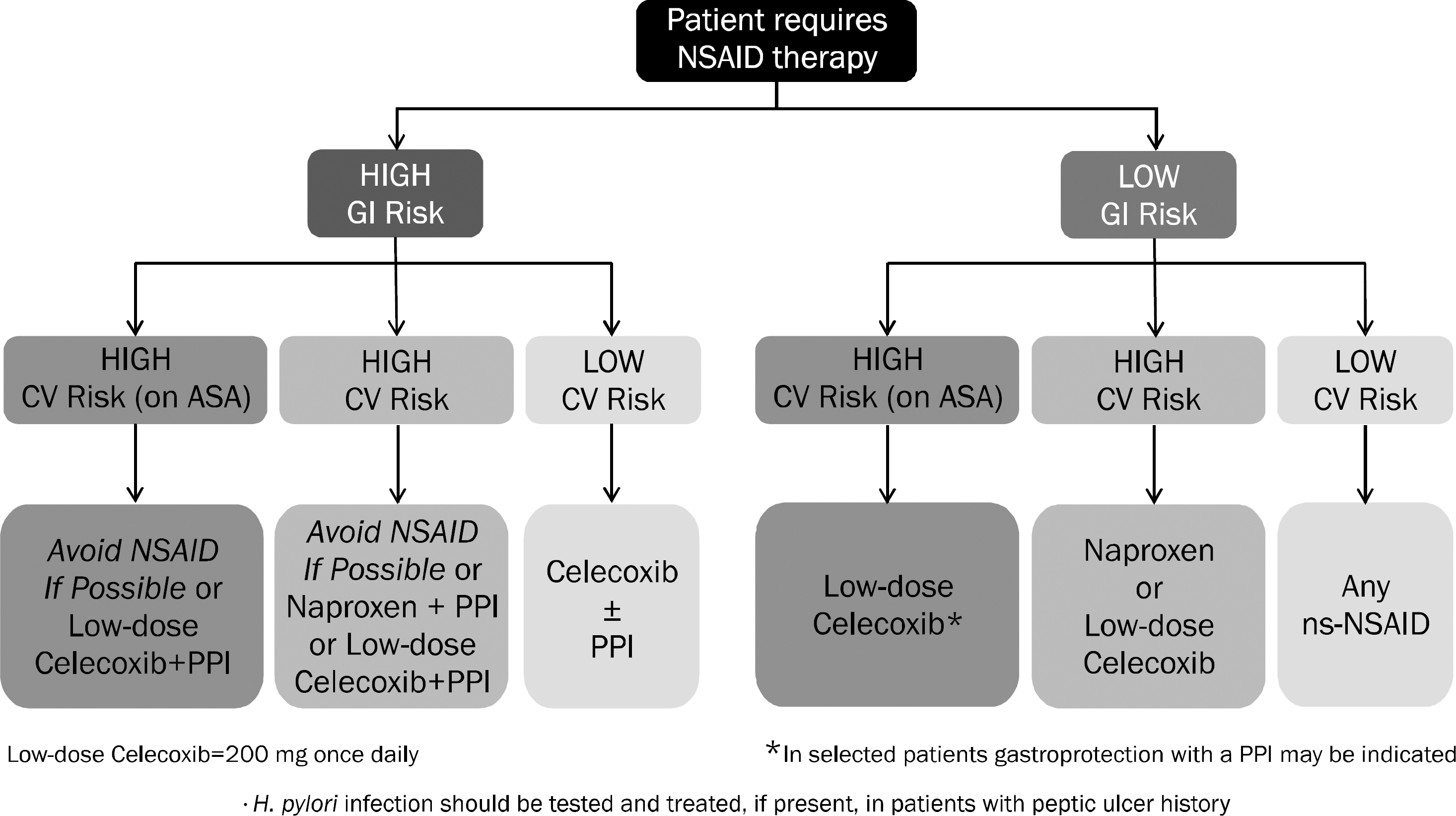
Table 1.
Changes of Clinical Characteristics of Patients with Peptic Ulcer in Korea
| Year | 199019 | 19956 | 199619 | 1996–199720 | 20006 | 2003–200818 | 20056 | 200619 | 200722 |
|---|---|---|---|---|---|---|---|---|---|
| Patient (n) | 60 | 1,518 | 80 | 180 | 1,980 | 475 | 2,042 | 61 | 310 |
| Age (yr), mean±SD | 47.8 | 50.8 | 53.8±13.7 | 58.2±14.9 | 58.1 | 61.5±15.0 | |||
| Aged patients (%) | 32.2 (≥60 yr) | 29.6 (>70 yr) | 48.1 (>65 yr) | ||||||
| NSAID and ulcerogenic drugs a (%) | 26.1 | 23.6 (NSAID),22.5 (aspirin) | 28.0 | 21.0 | |||||
| Helicobacter pylori infection (%) | 68.1 | 82.8 | 59.7 | 72.6 | 57.2 | 48.0 | |||
| Male (%) | 82.6 | 75.4 | 77.2 | 70.3 | 66.7 | 66.7 | |||
| Location of ulcer | |||||||||
| Gastric ulcer (%) | 52.4 | 53.3 | 57.3 | 59.1 | 56.0 | 56.0 | 52.0 | 61.2 | |
| Duodenal ulcer (%) | 40.4 | 46.7 | 44.4 | 40.9 | 44.0 | 44.0 | 40.7 | 38.8 | |
| Smoking (%) | 58.9 | 34.3 | |||||||
| Alcohol (%) | 43.9 | 35.8 | |||||||
| Bleeding (%) | 17.8 | 35.5 |
Table 2.
Demographic Characteristics of Korean Patients with Peptic Ulcer Bleeding and Perforated Peptic Ulcer in 2006–20077,8
| Variable |
Peptic ulcer bleeding (n=21,107)7 |
Perforated peptic ulcer (n=4,258)8 |
||||
|---|---|---|---|---|---|---|
| n (%) | 30-day mortality No. of deaths (%) | Crude MRR (95% CI) | n (%) | 30-day mortality No. of deaths (%) | Crude MRR (95% CI) | |
| Total | 21,107 (100) | 454 (2.2) | 4,258 | 135 (3.2) | ||
| Age (yr) | ||||||
| <60 | 11,099 (52.6) | 92 (0.8) | 1.00 | 3,143 (73.8) | 33 (1.1) | 1.00 |
| 60–79 | 8,453 (40.0) | 243 (2.9) | 3.50 (2.75–4.45) | 892 (20.9) | 59 (6.6) | 2.76 (1.7–4.5) |
| ≥80 | 1,555 (7.4) | 119 (7.7) | 9.55 (7.28–12.5) | 223 (5.2) | 43 (19.3) | 8.39 (4.8–14.1) |
| Sex | ||||||
| Male | 16,177 (76.6) | 296 (1.8) | 1.00 | 3,650 (85.7) | 74 (2.0) | 1.00 |
| Female | 4,930 (23.4) | 158 (3.2) | 1.78 (1.46–2.16) | 608 (14.3) | 61 (10.0) | 1.71 (1.1–2.6) |
| Charlson comorbidity index | ||||||
| Low (0) | 19,779 (93.7) | 366 (1.9) | 1.00 | 3,291 (77.3) | 38 (1.2) | 1.00 |
| Medium (1–2) | 1,158 (5.5) | 64 (5.5) | 3.53 (2.75–4.53) | 747 (17.5) | 60 (8.0) | 3.85 (2.5–6.0) |
| High (≥3) | 170 (0.8) | 14 (8.2) | 4.62 (2.71–7.88) | 220 (5.2) | 37 (16.8) | 8.52 (5.1–14.3) |
| PU-related hospitalization | ||||||
| No | 20,230 (95.8) | 427 (2.1) | 1.00 | 4,053 (95.2) | 118 (2.9) | Excluded c |
| Yes | 877 (4.2) | 27 (3.1) | 1.47 (0.99–2.19) | 205 (4.8) | 17 (8.3) | |
| Ulcer-related drug a users | ||||||
| No | 15,605 (73.9) | 301 (1.9) | 1.00 | 2,710 (63.6) | 43 (1.6) | Excluded c |
| Yes | 5,502 (26.1) | 153 (2.8) | 1.45 (1.19–1.77) | 1,548 (36.4) | 92 (5.9) | |
| Antiulcer drug b users | ||||||
| No | 8,294 (39.3) | (1.9) d | 1.00 | 3,602 (84.6) | 134 (3.7) | Excluded c |
| Yes | 12,813 (60.7) | (2.8) d | 1.45 (1.19–1.77) | 656 (15.4) | 1 (0.2) | |
MRR, mortality rate ratio; PU, peptic ulcer; PUB, peptic ulcer bleeding; PPU, perforated peptic ulcer.
a Ulcer-related drug is defined as NSAIDs (including aspirin and COX-2 inhibitors), oral glucocorticoids, and anticoagulant (warfarin and clopidogrel).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download