Abstract
Although, the prevalence of Helicobacter pylori infection in Korea has declined owing to the eradication therapy, recent seroprevalence of H. pylori infection is still reported to be as high as 54.4%. Until now, “standard regimen” for eradication of H. pylori has been conventional triple therapy consisting of proton pump inhibitor, amoxicillin, and clarithromycin. However, with the increase in antibiotic resistance, especially against clarithromycin, the eradication rate of conventional triple therapy has steadily declined during the past 13 years in Korea. Present eradication rate of standard triple therapy is reported to be less than 80%, which is the Maginot line of efficacy for the currently available regimen. Therefore, new first line eradication regimen is needed to enhance the eradication rate of H. pylori infection.
References
1. Franchini M, Cruciani M, Mengoli C, Pizzolo G, Veneri D. Effect of Helicobacter pylori eradication on platelet count in idiopathic thrombocytopenic purpura: a systematic review and metaanalysis. J Antimicrob Chemother. 2007; 60:237–246.
2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.
3. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

4. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.
5. Malfertheiner P, Mégraud F, O'Morain C, et al. Current European concepts in the management of Helicobacter pylori infection–the Maastricht Consensus Report. The European Helicobacter pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997; 9:1–2.
6. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
7. Kim SG, Jung HK, Lee HL, et al. Korean College of Helicobacter and Upper Gastrointestinal Research. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
8. Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999; 4:135–139.
9. European Helicobacter pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997; 41:8–13.
10. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007; 12:275–278.
11. Bochenek WJ, Peters S, Fraga PD, et al. Helicobacter pylori Pantoprazole Eradication (HELPPE) Study Group. Eradication of Helicobacter pylori by 7-day triple-therapy regimens combining pantoprazole with clarithromycin, metronidazole, or amoxicillin in patients with peptic ulcer disease: results of two double-blind, randomized studies. Helicobacter. 2003; 8:626–642.
12. Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004; 20:99–107.
13. Della Monica P, Lavagna A, Masoero G, Lombardo L, Crocellá L, Pera A. Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther. 2002; 16:1269–1275.
14. Higuchi K, Maekawa T, Nakagawa K, et al. Efficacy and safety of Helicobacter pylori eradication therapy with omeprazole, amoxicillin and high- and low-dose clarithromycin in Japanese patients: a randomised, double-blind, multicentre study. Clin Drug Investig. 2006; 26:403–414.
15. Kim BG, Lee DH, Ye BD, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007; 12:31–35.

16. Zhang L, Shen L, Ma JL, et al. Eradication of H pylori infection in a rural population: one-day quadruple therapy versus 7-day triple therapy. World J Gastroenterol. 2006; 12:3915–3918.
17. Kim SY, Jung SW. Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2011; 58:67–73.
18. Cho DK, Park SY, Kee WJ, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol. 2010; 55:368–375.
19. Choi YS, Cheon JH, Lee JY, et al. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006; 48:156–161.
20. Chung JW, Lee GH, Han JH, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.
21. Chung WC, Lee KM, Paik CN, et al. Inter-departmental differences in the eradication therapy for Helicobacter pylori infection: a single center study. Korean J Gastroenterol. 2009; 53:221–227.
22. Na HS, Hong SJ, Yoon HJ, et al. Eradication rate of first-line and second-line therapy for Helicobacter pylori infection, and reinfection rate after successful eradication. Korean J Gastroenterol. 2007; 50:170–175.
23. An B, Moon BS, Kim H, et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med. 2013; 33:415–419.
24. Kim SY, Lee SW, Jung SW, et al. Comparative study of Helicobacter pylori eradication rates of twice-versus four-times- daily amoxicillin administered with proton pump inhibitor and clarithromycin: a randomized study. Helicobacter. 2008; 13:282–287.
25. Jang HJ, Choi MH, Kim YS, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005; 46:368–372.
26. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.
27. Park HG, Jung MK, Jung JT, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012; 35:56–65.
28. Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011; 34:1098–1105.
29. Choi HS, Chun HJ, Park SH, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012; 18:2377–2382.
30. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.
31. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.

32. Song JG, Lee SW, Park JY, et al. Trend in the eradication rates of Helicobacter pylori infection in the last 11 years. Korean J Med. 2009; 76:303–310.
33. Cho HJ, Bae RC, Lee SH, et al. The trend in the eradication rates of first- and second-line therapy for Helicobacter pylori infection in Daegu and Kyungpook provinces: a single center experience for the most recent 9 years. Korean J Med. 2009; 76:186–192.
34. Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013; 47:21–24.
35. Kim SY, Jung SW, Kim JH, et al. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br J Clin Pharmacol. 2012; 73:140–143.
36. Kim JY, Kim N, Park HK, et al. Primary antibiotic resistance of Helicobacter pylori strains and eradication rate according to gastroduodenal disease in Korea. Korean J Gastroenterol. 2011; 58:74–81.
37. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy–the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999; 13:1047–1055.
38. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
39. Jung HS, Shim KN, Park H, et al. Meta-analysis of the H. pylori eradication rates according to the duration of the first-line therapy. Korean J Helicobacter Up Gastrointest Res. 2008; 8:9–14.
40. Mégraud F, Lehours P. Helicobacter pylori detection and anti-microbial susceptibility testing. Clin Microbiol Rev. 2007; 20:280–322.
41. Georgopoulos S, Papastergiou V, Xirouchakis E, et al. Nonbismuth quadruple "concomitant" therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J Clin Gastroenterol. 2013; 47:228–232.
42. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013; 145:121–128.e1.
Fig. 1.
The decreasing trend of Helicobacter pylori eradication rate with conventional triple therapy in Korea.
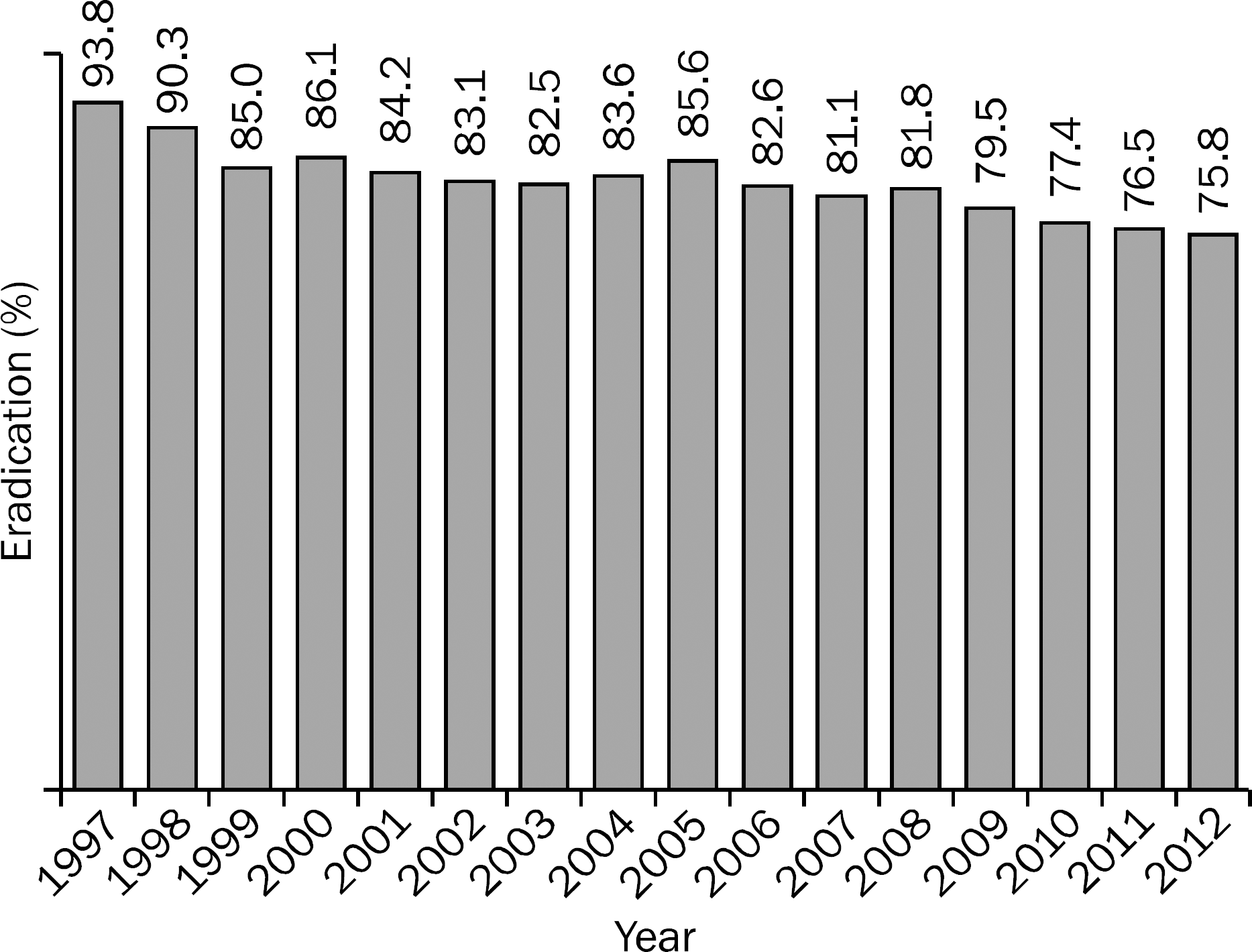
Table 1.
Recent Studies Evaluating Conventional Triple Therapy for Eradication of Helicobacter pylori Infection in Korea
| First author | Year of publication | Study duration | Study design | Treatmenta | Duration of treatment (day) | Patient (n) | Eradication rate (ITT, %) | Eradication rate (PP, %) |
|---|---|---|---|---|---|---|---|---|
| Jang25 | 2005 | 2003–2004 | RCT | PPI+ A 1,000 mg+ C 500 mg | 7 | 75 | 78.7 | 85.5 |
| Choi19 | 2006 | 1998–2005 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 | 525 | 78.7 | |
| Kim15 | 2007 | 2002–2003 | RCT | O 20 mg+ A 1,000 mg+ C 500 mg | 7 | 337 | 71.2 | 83.6 |
| Na22 | 2007 | 2001–2006 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 | 3,267 | 84.5 | |
| Kim24 | 2008 | 2005–2006 | RCT | O 20 mg+ A 1,000 mg+ C 500 mg | 14 | 93 | 91.4 | 92.1 |
| Choi26 | 2008 | 2007 | RCT | O 20 mg+ A 1,000 mg+ C 500 mg | 7 | 81 | 71.6 | 76.6 |
| Song32 | 2009 | 1997–2007 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 or 14 | 1,789 | 85.5 | |
| Cho33 | 2009 | 1999–2007 | Retro | PPI+ A 1,000 mg+ C 500 mg | − | 615 | 81.6 | |
| Chung21 | 2009 | 2003–2007 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 | 1,716 | 82.5 | |
| Cho18 | 2010 | 2001–2009 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 | 709 | 77 | |
| Kim28 | 2011 | 2008–2009 | RCT | P 40 mg+ A 1,000 mg+ C 500 mg | 14 | 204 | 75 | 85 |
| Kim36 | 2011 | 2006–2010 | Retro | PPI+ A 1,000 mg+ C 500 mg | 7 | 120 | 89.2 | |
| Choi29 | 2012 | 2008–2011 | RCT | R 20 mg+ A 1,000 mg+ C 500 mg | 7 | 115 | 70.4 | 75.7 |
| R 20 mg+ A 1,000 mg+ C 500 mg | 10 | 115 | 74.7 | 81.9 | ||||
| R 20 mg+ A 1,000 mg+ C 500 mg | 14 | 115 | 80 | 84.4 | ||||
| Chung30 | 2012 | 2010–2011 | RCT | L 30 mg+ A 1,000 mg+ C 500 mg | 10 | 80 | 58.7 | 67.6 |
| Kim35 | 2012 | 2009–2010 | RCT | L 30 mg+ A 1,000 mg+ C 500 mg | 14 | 104 | 74 | 82.8 |
| Oh31 | 2012 | 2009–2010 | RCT | R 20 mg+ A 1,000 mg+ C 500 mg | 7 | 130 | 63 | 64.5 |
| Park27 | 2012 | 2009–2010 | RCT | R 20 mg+ A 1,000 mg+ C 500 mg | 7 | 164 | 62.2 | 76 |
| An23,b | 2013 | 2009–2012 | Retro | P or E 30 mg+ A 2,250 mg+ C 1,000 mg | g 7 | 66 | 75.8 | |
| Kim34 | 2013 | 2009–2010 | RCT | L 30 mg+ A 1,000 mg+ C 500 mg | 7 | 135 | 72.6 | 85.2 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download