Abstract
Background/Aims
Conventional triple therapy (CT) for Helicobacter pylori infection fails in up to one-third of patients. Sequential therapy (ST) seem be more effective than CT in other countries. However, there is no systemic literature review that directly compares CT and ST in Korea. The aim of this study was to compare ST with CT for H. pylori infection in Korea.
Methods
Six randomized, prospective controlled trials were used to compare 10-day ST and 7- to 14-day CT in treatmentnaive patients with documented H. pylori infection in Korea. Pooled eradication rates and OR with 95% CI were calculated.
Results
The intention-to-treat eradication rates of H. pylori involving 1,529 patients were 79.7% (95% CI, 76.8–82.5%) for ST (n=754) and 68.1% (95% CI, 64.8–71.4%) for CT (n=775) (OR, 1.838; p<0.001). The per-protocol eradication rate of H. pylori involving 1,366 patients was 86.4% (95% CI, 83.3–88.5%) for ST (n=682) and 76.0% (95% CI, 72.8–79.2%) for CT (n=684) (OR, 1.974; p<0.001).
References
1. Hentschel E, Brandstätter G, Dragosics B, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993; 328:308–312.
2. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102:1808–1825.
3. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Conference. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.
4. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
5. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.
6. Chung JW, Lee GH, Han JH, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011; 58:246–250.
7. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.
8. Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and metaanalysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009; 104:3069–3079.
9. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008; 148:923–931.
10. Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011; 378:507–514.
11. Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011; 34:1098–1105.
12. Park HG, Jung MK, Jung JT, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012; 35:56–65.
13. Choi WH, Park DI, Oh SJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008; 51:280–284.
14. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.

15. Choi HS, Chun HJ, Park SH, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012; 18:2377–2382.
16. Chung JW, Jung YK, Kim YJ, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012; 27:1675–1680.
17. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.

18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

19. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007; 12:275–278.
20. Sirimontaporn N, Thong-Ngam D, Tumwasorn S, Mahachai V. Ten-day sequential therapy of Helicobacter pylori infection in Thailand. Am J Gastroenterol. 2010; 105:1071–1075.
21. Sánchez-Delgado J, Calvet X, Bujanda L, Gisbert JP, Titó L, Castro M. Ten-day sequential treatment for Helicobacter pylori eradication in clinical practice. Am J Gastroenterol. 2008; 103:2220–2223.
22. Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003; 17:719–726.
23. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007; 146:556–563.
24. Malfertheiner P, Bazzoli F, Delchier JC, et al. Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011; 377:905–913.
25. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010; 8:36–41.
26. Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011; 16:139–145.
27. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011; 17:3971–3975.
28. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004; 48:4843–4847.
29. Chung JW, Lee GH, Jeong JY, et al. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol. 2012; 27:493–497.
Fig. 1.
Forest plot treated with sequential or triple therapy for intention-to-treat (ITT) eradication rate. ST, sequential therapy; CT, conventional triple therapy.
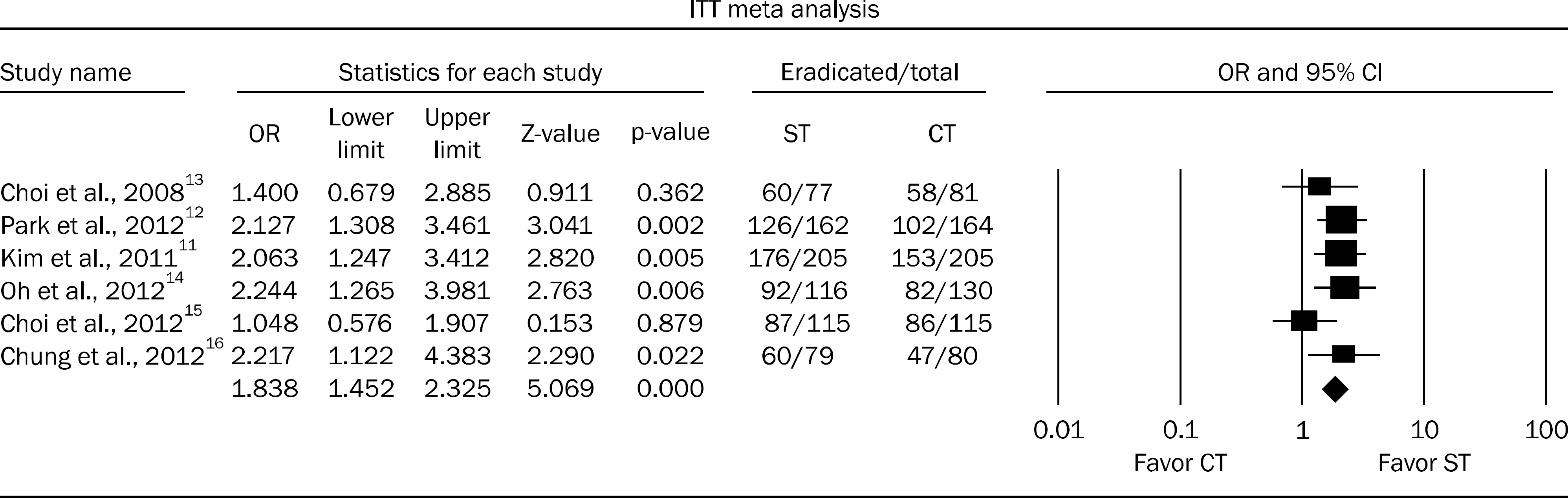
Fig. 2.
Forest plot treated with sequential or triple therapy for per-protocol (PP) eradication rate. ST, sequential therapy; CT, conventional triple therapy.
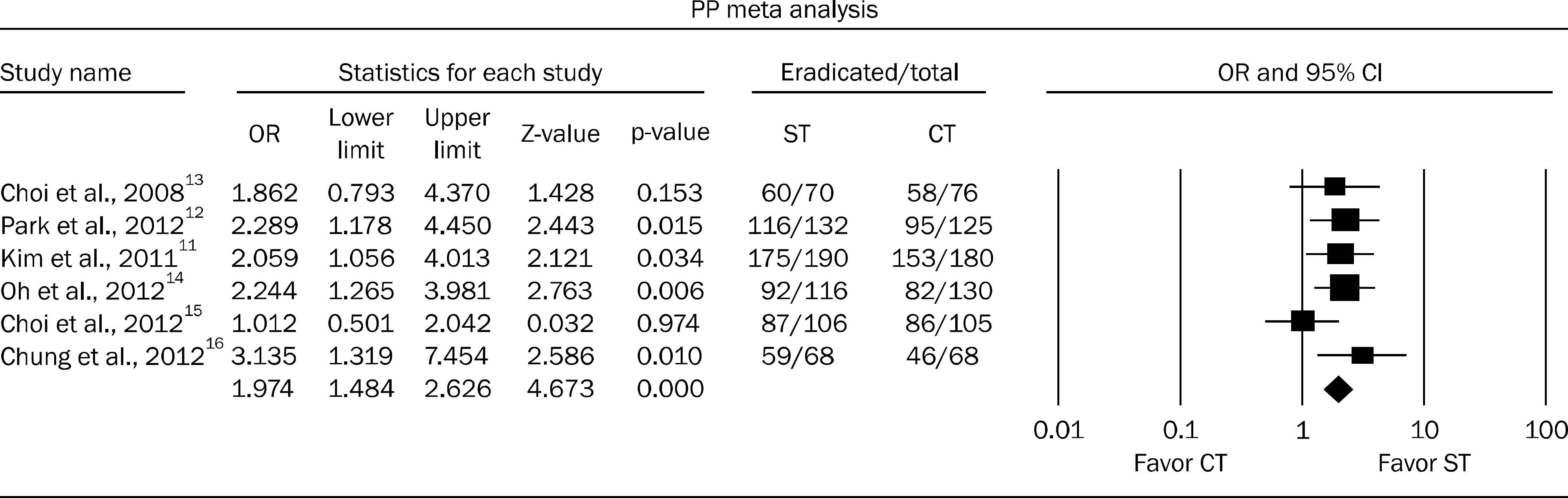
Table 1.
Studies of the Efficacy of Sequential Therapy for Treatment of Helicobacter pylori Infection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download