Abstract
Background/Aims
There is a paucity of national guideline for colorectal cancer screening and polyp diagnosis in Korea. Thus, we investigated the present state of colorectal cancer screening and polyp diagnosis methods using web-based survey to use as reference data for developing a guideline.
Methods
A multiple choice questionnaires of screening recommendations was sent via e-mail to members of the Korean Association for the Study of Intestinal Diseases and primary care physicians who participated in the national colonoscopy surveillance program. Among 425 colonoscopists, a total 263 colonoscopists replied (response rate, 61.9%).
Results
The most commonly recommended starting age for colorectal cancer screening and polyp diagnosis was 50 years old in the average risk group, and 40 years old in groups who had a family history of colon cancer (64.3% and 65.0% respectively). Surgeons had a tendency to recommend screening in younger people than internist do. Ninety-eight percent of physicians recommended screening colonoscopy to asymptomatic, average-risk patients as a first choice. Only 2% of physicians chose sigmoidoscopy as a screening tool. When the initial colonoscopy showed a negative finding, over 60% of internists repeated the exam 5 years later, whereas 62% of surgeons did so within 3 years.
Conclusions
The starting age of colorectal cancer screening and the interval of the colorectal polyp examination are not uniform in various medical environments, and there is a discrepancy between the practical recommendations and western guidelines. Thus, a new evidence-based national practice guideline for colorectal cancer screening and polyp diagnosis should be developed.
Figures and Tables
Fig. 1
Questionnaire about physician's perceptions and recommendations for colorectal cancer/polyp screening.

Fig. 2
The most influential guideline in practice. KSCP/NCC guideline was a most referenced guideline by physicians in this survey. The next influential guideline was ASGE guideline.
KSCP, Korean Society of Coloproctology; NCC, National Cancer Center; ASGE, American Society of Gastrointestinal Endoscopy; USMSTF, US Multi-Society Task Force; ACS, American Cancer Society; ACG, American College of Gastroenterology; BSG, British Society of Gastroenterology; EPAGE, European Panel on the Appropriateness of Gastrointestinal Endoscopy.
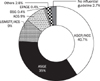
Fig. 3
Physician's perceptions of test performance for colorectal polyp diagnosis (n=263). Eighty-four percent of physicians recognized colonoscopy was a very effective screening tool for colorectal cancer/polyp, while FOBT was recognized as an ineffective screening tool by 57% of physicians.
DCBE, double contrast barium enema; FOBT, fecal occult blood test.

Fig. 4
Physician's modality-specific recommendations for colorectal polyp screening (n=263). Multiple choices were allowed. Ninety-eight percent of physicians recommend screening colonoscopy to asymptomatic, average-risk patients as a first choice. Only 2% of physicians choose sigmoidoscopy as a screening tool.
CFS, colonoscopy; CTC, CT colonography; FOBT, fecal occult blood test; Sig, Sigmoidoscoy; DCBE, double contrast barium enema.
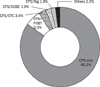
Fig. 5
Physicians' action for a detected polyp at screening colonoscopy. For polyps smaller than 0.5 cm, 84% of physicians removed them immediately after the detection using biopsy forcep. For polyps larger than 1.0 cm, 54.4% of physicians removed them with one-stage polypectomy, 38.4% with two-stage polypectomy.

References
1. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Shin HR, Won YJ, Jung KW, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005. 37:325–331.
3. Winawer S, Fletcher R, Rex D, et al. Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003. 124:544–560.
4. Lee BH, Jeong SY. Korean National Recommendation Guidelines on Screening and Surveillance for Early Detection of Colorectal Cancers. J Korean Med Assoc. 2002. 45:981–991.
5. Hong SN, Yang DH, Kim YH, et al. Multi-society task force for the guidelines for colorectal polyp screening, surveillance and management. A Survey for post-polypectomy surveillance. Intest Res. 2011. 9:118–128.
6. Arditi C, Peytremann-Bridevaux I, Burnand B, et al. EPAGE II Study Group. Appropriateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy. 2009. 41:200–208.
7. Davila RE, Rajan E, Baron TH, et al. Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006. 63:546–557.
8. Bond JH. Practice Parameters Committee of the American College of Gastroenterology. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Am J Gastroenterol. 2000. 95:3053–3063.
9. Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008. 134:1570–1595.
10. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009. 104:739–750.
11. U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Am Fam Physician. 2002. 66:2287–2290.
12. Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010. 105:2017–2025.
13. Lee SH, Lee KS, Lee JY, et al. Clinical usefulness of fecal occult blood test as a screening method for asymptomatic patients with colon polyps. Korean J Gastroenterol. 2006. 48:388–394.
14. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005. 129:422–428.
15. Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008. 359:1207–1217.
16. Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008. 249:151–159.
17. Gluecker TM, Johnson CD, Wilson LA, et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003. 124:911–916.
18. Lewis JD, Ng K, Hung KE, et al. Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med. 2003. 163:413–420.
19. Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Med Inform Decis Mak. 2008. 8:4.
20. Atkin WS, Edwards R, Kralj-Hans I, et al. UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010. 375:1624–1633.
21. Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006. 295:2366–2373.
22. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007. 132:96–102.
23. Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008. 6:1117–1121.
24. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009. 150:1–8.
25. Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002. 346:1781–1785.
26. Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008. 300:1417–1422.
27. Betés M, Muñoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003. 98:2648–2654.
28. Choe JW, Chang HS, Yang SK, et al. Screening colonoscopy in asymptomatic average-risk Koreans: analysis in relation to age and sex. J Gastroenterol Hepatol. 2007. 22:1003–1008.
29. Lieberman D. Endoscopic colon screening: is less more? Gastroenterology. 1996. 111:1385–1387.
30. Sung JJ, Lau JY, Young GP, et al. Asia Pacific Working Group on Colorectal Cancer. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008. 57:1166–1176.
31. Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008. 58:130–160.
32. Morson BC. Evolution of cancer of the colon and rectum. Cancer. 1974. 34:Suppl. 845–849.
33. Kato H, Haga S, Endo S, et al. Lifting of lesions during endoscopic mucosal resection (EMR) of early colorectal cancer: implications for the assessment of resectability. Endoscopy. 2001. 33:568–573.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download