Abstract
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma represents approximately 40% of gastric lymphomas, and its incidence is increasing. An early diagnosis for gastric MALT lymphoma is important, but not easy due to non-specific symptoms and endoscopic findings. Diagnosis is based on the histopathologic evaluation of multiple, deep and repeated biopsies taken from normal and any abnormal appearing sites of the stomach. In addition, the presence of Helicobacter pylori (H. pylori) infection must be determined to determine therapeutic approach. Endoscopic ultrasonography (EUS) is essential for the evaluation of regional lymph nodes and the depth of tumor invasion in the gastric wall, for predicting response to H. pylori eradication, and for monitoring tumor regression or recurrence. The eradication of H. pylori is recommended as an initial treatment for low-grade gastric MALT lymphoma with H. pylori infection. Both radiation therapy and chemotherapy are suitable alternative options for H. pylori-negative, refractory, or high-grade gastric MALT lymphoma. But, the role of surgery is diminishing. After treatment, strict endoscopic regular follow-up including EUS is recommended with multiple biopsies. However, controversy remains regarding the best diagnosis, treatment and follow-up strategy for this disease.
References
1. Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001; 19:3861–3873.

2. Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin's lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma. 2006; 47:2140–2146.

3. Ferrucci P, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007; 136:521–538.
4. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program;2009. p. 523–531.
5. Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
6. Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000; 96:410–419.

7. Lee HY, Kim JJ, Phee PL, et al. Endoscopic findings of gastric mu-cosa-associated lymphoid tissue (MALT) lymphoma. Korean J Gastrointest Endosc. 1997; 17:125–131.
9. Taal BG, den Hartog Jager FC, Tytgat GN. The endoscopic spectrum of primary non-Hodgkin's lymphoma of the stomach. Endoscopy. 1987; 19:190–192.

10. Chin YJ, Chang DK, Lee KM, et al. Clinicopathologic study of primary gastric lymphoma of B-cell phenotype with special reference to low-grade B-cell lymphoma of MALT. Korean J Gastroenterol. 1998; 31:463–476.
11. Yokoi T, Nakamura T, Nakamura S. Differential diagnosis of gastric MALT lymphomas. Stomach Intest. 2001; 36:13–20.
12. Seifert E, Schulte F, Weismüller J, de Mas CR, Stolte M. Endoscopic and bioptic diagnosis of malignant non-Hodgkin's lymphoma of the stomach. Endoscopy. 1993; 25:497–501.

13. Caletti G, Barbara L. Gastric lymphoma: difficult to diagnose, difficult to stage? Endoscopy. 1993; 25:528–530.

14. Taal BG, Boot H, van Heerde P, de Jong D, Hart AA, Burgers JM. Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept. Gut. 1996; 39:556–561.

15. Hummel M, Oeschger S, Barth TF, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006; 55:782–787.

16. Mehra M, Agarwal B. Endoscopic diagnosis and staging of mucosa-associated lymphoid tissue lymphoma. Curr Opin Gastroenterol. 2008; 24:623–626.

17. Toyoda H, Ono T, Kiyose M, et al. Gastric mucosa-associated lymphoid tissue lymphoma with a focal high-grade component diagnosed by EUS and endoscopic mucosal resection for histologic evaluation. Gastrointest Endosc. 2000; 51:752–755.

18. Queneau PE, Helg C, Brundler MA, et al. Diagnosis of a gastric mucosa-associated lymphoid tissue lymphoma by endoscopic ultrasonography-guided biopsies in a patient with a parotid gland localization. Scand J Gastroenterol. 2002; 37:493–496.

19. Fujishima H, Misawa T, Maruoka A, Chijiiwa Y, Sakai K, Nawata H. Staging and follow-up of primary gastric lymphoma by endoscopic ultrasonography. Am J Gastroenterol. 1991; 86:719–724.
20. Lévy M, Hammel P, Lamarque D, et al. Endoscopic ultrasonography for the initial staging and follow-up in patients with low-grade gastric lymphoma of mucosa-associated lymphoid tissue treated medically. Gastrointest Endosc. 1997; 46:328–333.

21. Caletti G, Ferrari A, Brocchi E, Barbara L. Accuracy of endoscopic ultrasonography in the diagnosis and staging of gastric cancer and lymphoma. Surgery. 1993; 113:14–27.
22. Palazzo L, Roseau G, Ruskone-Fourmestraux A, et al. Endoscopic ultrasonography in the local staging of primary gastric lymphoma. Endoscopy. 1993; 25:502–508.

23. Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol. 2003; 98:975–986.

24. Lee CR, Cho YS, Cheong JY, et al. The role of endoscopic ultrasonography in primary gastric lymphoma of MALT. Korean J Gastrointest Endosc. 1999; 19:869–877.
25. Grau E, Gomez A, Cuñat A, Oltra C. Computed tomography in staging of primary gastric lymphoma. Lancet. 1996; 347:1261.

26. Elstrom R, Guan L, Baker G, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003; 101:3875–3876.

27. Alinari L, Castellucci P, Elstrom R, et al. 18F-FDG PET in mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma. 2006; 47:2096–2101.

28. Lee MH, Jung HY, Kang GH, et al. Two cases of gastric low-grade mucosa-associated lymphoid tissue lymphoma involving bone marrow. Korean J Gastroenterol. 2000; 36:695–700.
29. Neubauer A, Thiede C, Morgner A, et al. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mu-cosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997; 89:1350–1355.
30. Nobre-Leitão C, Lage P, Cravo M, et al. Treatment of gastric MALT lymphoma by Helicobacter pylori eradication: a study controlled by endoscopic ultrasonography. Am J Gastroenterol. 1998; 93:732–736.
31. Sackmann M, Morgner A, Rudolph B, et al. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. MALT Lymphoma Study Group. Gastroenterology. 1997; 113:1087–1090.
32. Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992; 102:1628–1638.

33. Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995; 345:1591–1594.
34. Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995; 122:767–769.
35. Pinotti G, Zucca E, Roggero E, et al. Clinical features, treatment and outcome in a series of 93 patients with low-grade gastric MALT lymphoma. Leuk Lymphoma. 1997; 26:527–537.

36. Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med. 1998; 49:289–299.
37. Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology. 1991; 101:1159–1170.

39. Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001; 48:297–303.
40. Nakamura S, Matsumoto T, Suekane H, et al. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001; 48:454–460.
41. Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001; 357:39–40.
42. Fung CY, Grossbard ML, Linggood RM, et al. Mucosa-associated lymphoid tissue lymphoma of the stomach: long term outcome after local treatment. Cancer. 1999; 85:9–17.
43. Hammel P, Haioun C, Chaumette MT, et al. Efficacy of single-agent chemotherapy in low-grade B-cell mucosa-associated lymphoid tissue lymphoma with prominent gastric expression. J Clin Oncol. 1995; 13:2524–2529.

44. Nakamura S, Matsumoto T, Suekane H, et al. Longterm clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer. 2005; 104:532–540.
45. Martinelli G, Laszlo D, Ferreri AJ, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol. 2005; 23:1979–1983.
46. Schechter NR, Yahalom J. Low-grade MALT lymphoma of the stomach: a review of treatment options. Int J Radiat Oncol Biol Phys. 2000; 46:1093–1103.

47. Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998; 16:1916–1921.

48. Nakamura S, Yao T, Aoyagi K, Iida M, Fujishima M, Tsuneyoshi M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer. 1997; 79:3–11.
49. Karat D, O'Hanlon DM, Hayes N, Scott D, Raimes SA, Griffin SM. Prospective study of Helicobacter pylori infection in primary gastric lymphoma. Br J Surg. 1995; 82:1369–1370.
50. Morgner A, Lehn N, Andersen LP, et al. Helicobacter heilman-nii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000; 118:821–828.

51. Ono H, Oda I, Inui T, et al. Clinical management for non-res-ponders with low-grade gastric MALT lymphoma after Helicobacter pylori eradication therapy. Stomach Intest. 2002; 37:521–529.
52. Wündisch T, Thiede C, Morgner A, et al. Longterm follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005; 23:8018–8024.
53. Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004; 53:34–37.
54. Urakami Y, Sano T, Begum S, Endo H, Kawamata H, Oki Y. Endoscopic characteristics of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2000; 15:1113–1119.
55. Ishihara R, Tatsuta M, Iishi H, Uedo N, Narahara H, Ishiguro S. Usefulness of endoscopic appearance for choosing a biopsy target site and determining complete remission of primary gastric lymphoma of mucosa-associated lymphoid tissue after eradication of Helicobacter pylori infection. Am J Gastroenterol. 2002; 97:772–774.
56. Montalban C, Manzanal A, Boixeda D, et al. Helicobacter pylori eradication for the treatment of low-grade gastric MALT lymphoma: follow-up together with sequential molecular studies. Ann Oncol. 1997; 8(Suppl 2):37–39.
57. Rudolph B, Bayerdörffer E, Ritter M, et al. Is the polymerase chain reaction or cure of Helicobacter pylori infection of help in the differential diagnosis of early gastric mucosa-associated lymphatic tissue lymphoma? J Clin Oncol. 1997; 15:1104–1109.
Fig. 1.
Endoscopic findings of superficial type gastric MALT lymphoma. (A) IIc-like type. (B) Submucosal tumor type. (C) Multiple erosion type.(D) Cobblestone-mucosa type. (E) Partial-fold-thickening type. (F) Discoloration type.
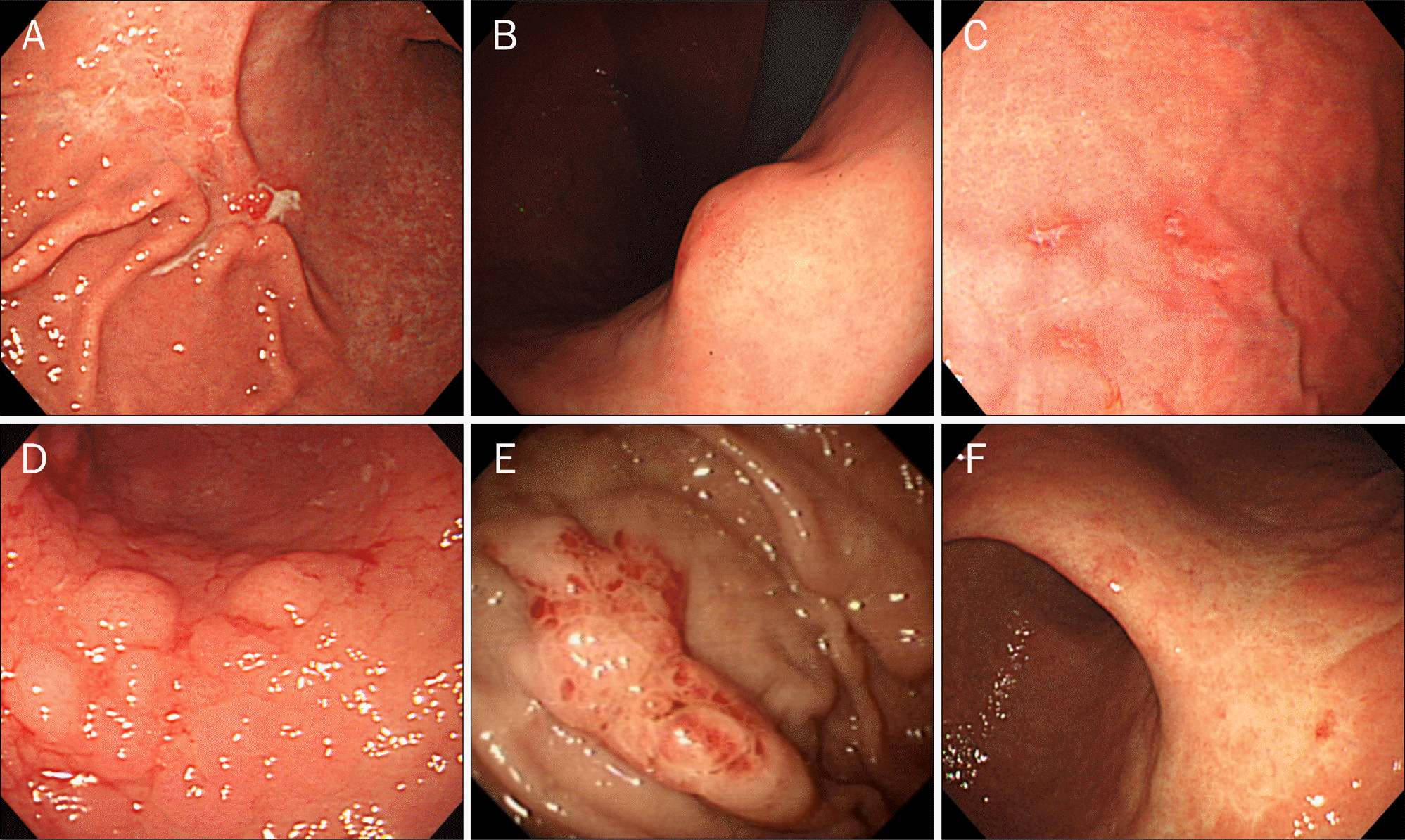
Fig. 2.
Histological features of gastric MALT lymphoma. Neoplastic lymphoid cells invaded gastric glands (lymphoepithelial lesion, arrow).
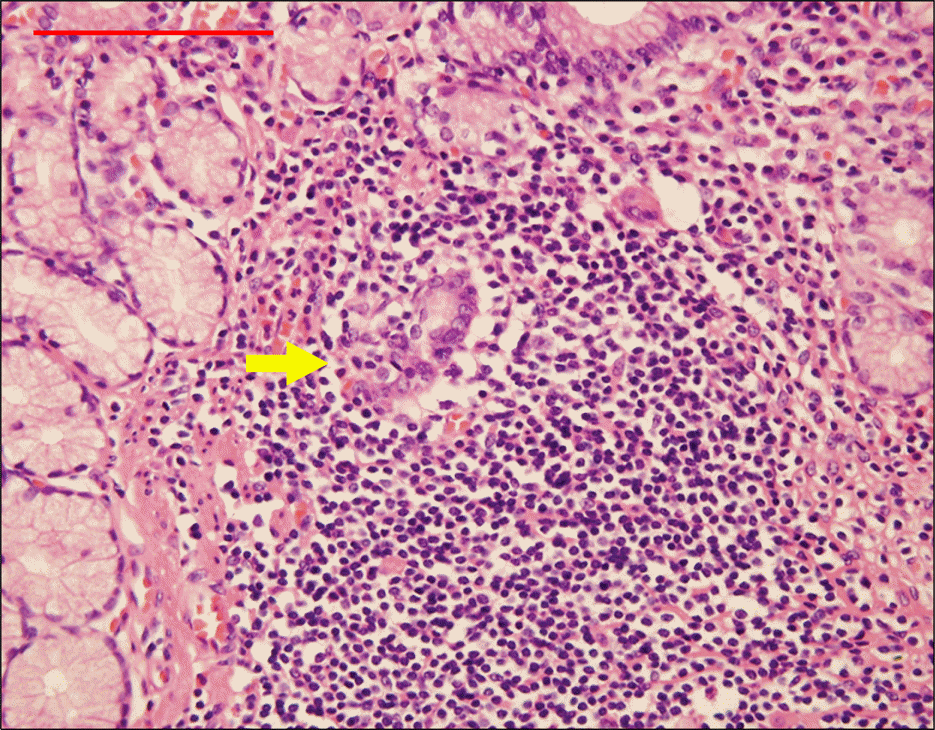
Fig. 3.
Algorithm suggested for the diagnostic procedure of gastric biopsies.15*Lymphoma of MALT type.
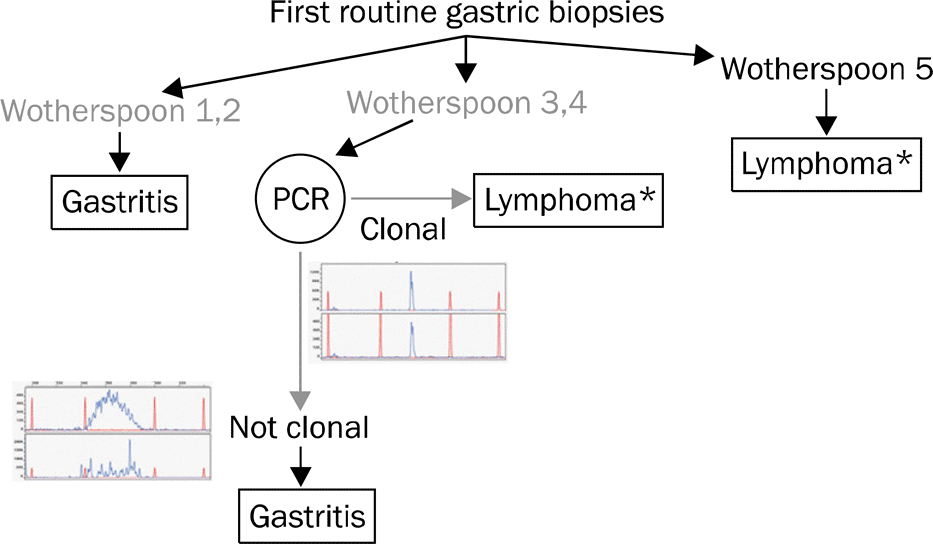
Fig. 4.
EUS findings of gastric MALT lymphoma. (A) Tumor limited to the mucosa (T1). (B) Tumor limited to the submucosa (T2). (C) Tumor extended to the serosa (T3).

Fig. 5.
Pre- and post-treatment endoscopic findings of gastric MALT lymphoma after H. pylori eradication. (A-C) Pre-treatment endoscopic findings. (D-F) Post-treatment endoscopic findings. Whitish discolored areas appeared at the site where the previous MALT lymphoma disappeared after H. pylori eradication.
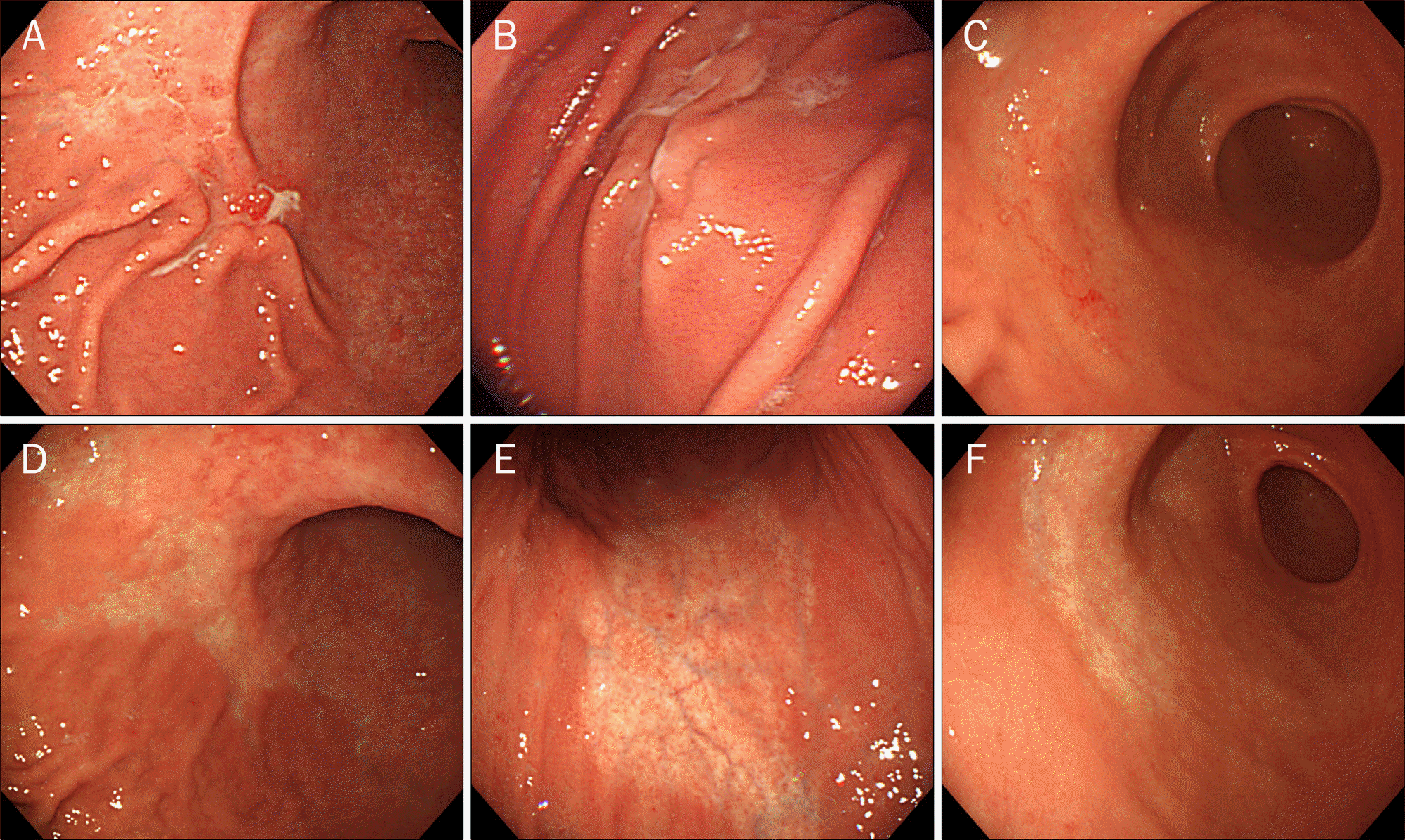
Table 1.
Low-grade Gastric MALT Lymphoma: Most Common Clinical Features6
Table 2.
Differential Points of Endoscopic Findings between EGC IIc and MALT Lymphoma
Table 3.
Histologic Scoring of Lymphoid Infiltrations in the Stomach5
Table 4.
EUS Staging of Gastric MALT Lymphoma
Table 5.
Ann Arbor Classification of Extranodal Lymphoma (Modified by Musshoff)23
Table 6.
Recommended Staging Procedures for Gastric Lymphoma6
Table 7.
Suggested Therapeutic Algorithm for Gastric MALT Lymphoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download